Abstract
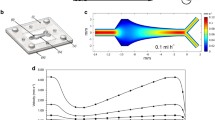
The relationship between biofilm formation and Reynolds number in laminar flow has been investigated usingPseudomonas fluorescens EX101. It was shown using a Modified Robbins Device that in laminar flow, numbers of viable cells in a developed biofilm increased with Reynolds number (Re 2, 17 and 51.5), as would be expected in a system where molecular transport to the wall is limited by diffusion. By monitoring fluorescent beads in a flowcell with a scanning confocal laser microscope at similar low Reynolds numbers, the velocity profile close to the solid surface was determined. It was shown that the presence of a thin bacterial film (up to 12 μm) displaced the flow profile away from the wall by a distance equivalent to the film thickness. Total cell counts from the Modified Robbins Device samples were not significantly different at the different flow rates but were higher than viable counts. Interruption of the flow had no significant effect on colonisation by the bacteria through the Modified Robbins Device in the first few hours. However, viable numbers were reduced when the flow was stopped at 7 h after initial colonisation.
Similar content being viewed by others
References
Block JC, K Houdidier, JL Paquin, J Miazga and Y Levi. 1993. Biofilm accumulation in drinking water distribution systems. Biofouling 6: 333–343.
Caldwell DE and JR Lawrence. 1986. Growth kinetics ofP. fluorescens microcolonies within the hydrodynamic boundary layers of surface microenvironments. Microb Ecol 12: 229–312.
Caldwell DE and JR Lawrence. 1989. Study of attached cells in continuous flow slide culture. In: A Handbook of Laboratory Model Systems for Microbial Ecosystem Research (Wimpenny JWT, ed), vol 1, pp 117–138, CRC Press, Boca Raton, FL.
Caldwell DE, DR Korber and JR Lawrence. 1992. Confocal laser microscopy and digital image analysis in microbial ecology. Adv Microb Ecol 12: 1–67.
Characklis WG, MH Turakhia and N. Zelver. 1990. Transport and interfacial transport phenomena. In: Biofilms (Characklis WG and KC Marshall, eds), pp 265–340, John Wiley and Sons, New York.
Cunningham AB. 1989. Hydrodynamics and solute transport at the fluid-biofilm interface. In: Structure and Function of Biofilms (Characklis WG and PA Wilderer, eds), pp 19–31, John Wiley and Sons, Chichester, UK.
Douglas JF, JM Gasiorek and JA Swaffield. 1984. Motion of fluid particles and streams. In: Fluid Mechanics. pp 97–117, Pitman Publishing Limited, London, UK.
Fletcher' M and KC Marshall. 1982. Are solid surfaces of ecological significance to aquatic bacteria? In: Advances in Microbial Ecology (Marshall KC, ed), vol 6, pp 199–236, Plenum Publishing Corp, New York.
Geesey GG and JW Costerton. 1981. Application of epifluorescence microscopy to the enumeration of aquatic bacteria concentrated on membrane filters. In: Membrane Filtration. Applications, Techniques and Problems (Dutka BJ, ed), pp 253–264, Marcel Dekker Inc, New York.
Huang CT, SW Peretti and JD Bryers. 1992. Use of flow cell reactors to quantify biofilm formation. Biotechnol Lett 6: 193–198.
Korber DR, JR Lawrence and DE Caldwell. 1994. Effect of motility on surface colonisation and reproductive success ofP. fluorescens in dual-dilution continuous culture and batch culture systems. Appl Environ Microbiol 60: 1421–1429.
Korber DR, JR Lawrence, L Zhang and DE Caldwell. 1990. Effect of gravity on bacterial deposition and orientation in laminar flow environments. Biofouling 2: 335–350.
Korber DR, GA James and JW Costerton. 1994. Evaluation of fleroxacin activity against establishedPseudomonas fluorescens biofilms. Appl Environ Microbiol 60: 1663–1669.
Lappin-Scott HM and JW Costerton. 1989. Bacterial biofilms and surface fouling. Biofouling 1: 323–342.
Lappin-Scott HM, J Jass and JW Costerton. 1993. Microbial biofilm formation and characterisation. In: Microbial Biofilms: Formation and Control (Denyer SP, SP Gorman and M Sussman, eds), pp 1–12, Blackwell Scientific Publications, UK.
Lappin-Scott HM, MG Brading and J Jass. 1994. How do bacteria reach surfaces? In: Bacterial Biofilms and their Control in Medicine and Industry (Wimpenny J, W Nichols, D Stickler and H Lappin-Scott, eds), pp 19–23, Bioline, UK.
Lau YL and D Liu. 1993. Effect of flow rate on biofilm accumulation in open channels. Water Res 27: 355–360.
Marshall KC. 1980. Bacterial adhesion in natural environments. In: Microbial Adhesion to Surfaces (Berkeley RCW, JM Lynch, J Melling, PR Rutter and B Vincent, eds), pp 187–196, Ellis Horwood Publishers, Chichester, UK.
Munson BR, DF Young and TH Okiishi. 1990. Viscous flow in pipes. In: Fundamentals of Fluid Mechanics. pp 465–559, John Wiley and Sons, New York.
Nickel JC, I Ruseska, JB White and JW Costeron. 1985. Tobramycin resistance ofPseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother 27: 619–624.
Powell MS and NKH Slater. 1983. The deposition of bacterial cells from laminar flows on to solid surfaces. Biotechnol Bioeng 25: 891–900.
Ramia M and D Tullock. 1990. Micro-organism locomotion: an application of fluid mechanics and kinematics. 5th International Conference on Rheology, Melbourne, 1990, pp 95–98.
Stoodley P, D DeBeer and Z Lewandowski. 1994. Liquid flow in biofilm systems. Appl Environ Microbiol 60: 2711–2716.
Walker JT, DE Caldwell, K Hanson and CW Keevil. 1994. Use of scanning confocal microscopy to study biofilm formation. In: Bacterial Biofilms and their Control in Medicine and Industry (Wimpenny J, W Nichols, D Stickler and H Lappin-Scott, eds), pp 69–76, Bioline, UK.
Watkins L and JW Costerton. 1984. Growth and biocide resistance of bacterial biofilms in industrial systems. Chemical Times and Trends 7(4): 35–40.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brading, M.G., Boyle, J. & Lappin-Scott, H.M. Biofilm formation in laminar flow usingPseudomonas fluorescens EX101. Journal of Industrial Microbiology 15, 297–304 (1995). https://doi.org/10.1007/BF01569983
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01569983