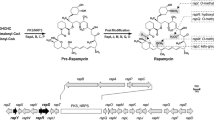
Summary
Although the addition of shikimic acid to the medium had no effect on the level of production of rapamycin byStreptomyces hygroscopicus,14C-shikimic acid was incorporated into rapamycin to a very high degree.13C-Shikimic acid was successfully prepared from 1-[13C]-glucose using a mutant ofKlebsiella pneumoniae, and used to label rapamycin. It was found that13C-shikimic acid was incorporated into the cyclohexane moiety of rapamycin, thereby establishing the shikimic acid pathway origin of the seven-carbon starter unit.
Similar content being viewed by others
References
Byrne, K.M., A. Shafiee, J.B. Nielsen, B. Arison, R.L. Monaghan and L. Kaplan. 1993. The biosynthesis and enzymology of an immunosuppressant, immunomycin, produced byStreptomyces hygroscopicus var.ascomycetius. In: Microbial Metabolites (Nash, C., J. Hunter-Cevera, R. Cooper, D.E. Eveleigh and R. Hamill, eds), pp. 29–45, Wm. C. Brown Publishers, Dubuque, Iowa.
Calne, R.Y., D.St.J. Collier, S. Lim, S.G. Pollard, A. Samann, D.J.G. White and S. Thiru. 1989. Rapamycin for immunosuppression in organ allografting. Lancet ii: 227.
Casati, R., J.M. Beale and H.G. Floss. 1987. Biosynthesis of ansatrienin. Nonincorporation of shikimic acid into the mC7N unit and stereochemistry of its conversion to the cyclohexanecarboxylic acid moiety. J. Am. Chem. Soc. 109: 8102–8104.
Cranswick, A.M. and J.A. Zabkiewicz. 1979. Quantitative analysis of monosaccharides, cyclitols, sucrose, quinic and shikimic acids inPinus radiata extracts on a glass support-coated open tubular capillary column by automated gas chromatography. J. Chromatog. 171: 233–242.
Degwert, U., R. Hulst, H. Pape, R.E. Herrold, J.M. Beale, P.J. Keller, J.P. Lee and H.G. Floss. 1987. Studies on the biosynthesis of the β-glucosidase inhibitor acarbose: valienamine, a m-C7N unit not derived from the shikimic pathway. J. Antibiot. 40: 855–861.
Douros, J. and M. Suffness. 1981. New antitumor substances of natural origin. Cancer Treat. Rev. 8: 63–87.
Dumont, F.J., M.J. Staruch, S.L. Koprak, M.R. Melino and N.H. Sigal. 1990. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J. Immunol. 141: 251–258.
Eng, C.P., S.N. Sehgal and C. Vezina. 1984. Activity of rapamycin (AY-22,989) against transplanted tumors. J. Antibiot. 37: 1231–1237.
Floss, H.A., H. Cho, R. Casati, K.A. Reynolds, E. Kennedy, B.S. Moore, J.M. Beale, U.M. Mocek and K. Poralla. 1992. Diversions of the shikimate pathway — the biosynthesis of cyclohexanecarboxylic acid. In: Secondary Metabolite Biosynthesis and Metabolism (Petroski, R.J. and S.P. McCormick, eds), pp. 77–88, Plenum Press, New York.
Ghisalba, O. and J. Nuesch. 1981. A genetic approach to the biosynthesis of the rifamycin-chromophore inNocardia mediterranei. IV. Identification of 3-amino-5-hydroxybenzoic acid as a direct precursor of the seven-carbon amino starter unit. J. Antibiot. 34: 64–71.
Gottschalk, G. 1979. Bacterial Metabolism. Springer-Verlag, New York.
Knowles, P.F. and D.B. Sprinson. 1970. Preparation of shikimate 5-phosphate. In: Methods in Enzymology, Vol. 17A (Tabor, H. and C.W. Tabor, eds), pp. 351–359, Academic Press, New York.
Martel, R.R., J. Klicus and S. Galet. 1977. Inhibition of the immune response by rapamycin, a new antifungal antibiotic. Can. J. Physiol. Pharmacol. 55: 48–51.
McAlpine, J.B., S.J. Swanson, M. Jackson and D.N. Whittern. 1991. Revised NMR assignments for rapamycin. J. Antibiot. 44: 688–690.
Millican, C.M. 1962. Biosynthesis of pyocyanine. Incorporation of14C-shikimic acid. Biochim. Biophys. Acta 57: 407–409.
Morris, R.E. and B.M. Meiser. 1989. Identification of a new pharmacologic action for an old compound. Med. Sci. Res. 17: 877–878.
Oshima, M., Y. Sakaki, T. Oshima. 1978. ω-Cyclohexyl fatty acids in acido-thermophilic bacterial membranes and phage capsids. In: Biochemistry of Thermophily (Friedman, S.M., ed.), pp. 31–44, Academic Press, New York.
Paiva, N.L., A.L. Demain and M.F. Roberts. 1991. Incorporation of acetate, propionate, and methionine into rapamycin byStreptomyces hygroscopicus. J. Nat. Prod. 54: 167–177.
Paiva, N.L., A.L. Demain and M.F. Roberts. 1993. The immediate precursor of the nitrogen-containing ring of rapamycin is free pipecolic acid. Enzyme Microb. Technol. 15: 581–585.
Rosen, M.K., R.F. Standaert, A. Galat, M. Nakatsuka and S.L. Schreiber. 1990. Inhibition of FKBP rotamase activity by immunosuppressant FK506: twisted amid surrogate. Science 248: 863.
Tanaka, H., A. Kuroda, H. Marusawa, H. Hatanaka, T. Kino, T. Goto, M. Hashimoto and T. Taga. 1987. Structure of FK506: a novel immunosuppressant isolated fromStreptomyces. J. Am. Chem. Soc. 109: 5031–5033.
Toyokuni, T., W.-Z. Jin and K.L. Rinehart, Jr. 1987. Biosynthetic studies on validamycins: A C2+C2+C3 pathway to an aliphatic C7N unit. J. Am. Chem. Soc. 109: 3481–3482.
Vezina, C., A. Kudelski and S.N. Sehgal. 1975. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. 28: 721–726.
Weiss, V. and E.S. Mingioli. 1956. Aromatic biosynthesis. 15. The isolation and identification of shikimate acid 5-phosphate. J. Am. Chem. Soc. 78: 2894–2898.
White, R.J. and E. Martinelli. 1974. Ansamycin biogenesis: Incorporation of [1-13C]glucose and [1-13C] glycerate into the chromophore of rifamycin S. FEBS Lett. 49: 233–236.
Yoshida, S. and M. Hasegawa. 1957. A microcolorimetric method for the determination of shikimic acid. Arch. Biochem. Biophys. 70: 377–381.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Paiva, N.L., Roberts, M.F. & Demain, A.L. The cyclohexane moiety of rapamycin is derived from shikimic acid inStreptomyces hygroscopicus . Journal of Industrial Microbiology 12, 423–428 (1993). https://doi.org/10.1007/BF01569676
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01569676