Summary
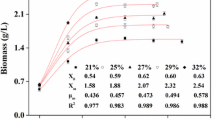
During the rapid growth phase ofStreptomyces clavuligerus in a 10 litre fermentor, the level of dissolved oxygen (DO) was found to drop to almost zero for a period of approximately 10 h, delaying the appearance of and lowering the production of the antibiotic cephamycin C. Controlling the DO at either 50% or 100% throughout the fermentation did not significantly alter the specific growth rate of the culture, but did elevate final antibiotic levels two- and three-fold respectively. The improved oxygen availability affected antibiotic production both by increasing the rate of specific cephamycin C bisosynthesis and by maintaining this higher rate throughout the production period. These results demonstrate that controlling dissolved oxygen levels close to saturation during periods of rapid growth markedly improves the efficiency and duration of cephamycin C biosynthesis inS. clavuligerus.
Similar content being viewed by others
References
Abraham, E.P., J.A. Huddleston, G.S. Jayatilake, J. O'Sullivan and R.L. White. 1981. Conversion of δ-(l-α-aminoadipyl)-l-cysteinyl-d-valine to isopenicillin N in cell-free extracts ofCephalosporium acremonium. In: Recent Advances in the Chemistry ofβ-lactam Antibiotics (2nd International Symposium) (Gregory, G.I., ed.), pp. 125–134, Special Publication, The Royal Society of Chemistry, London.
Agathos, S.N. and A.L. Demain. 1986. Dissolves and the in vivo stability of gramicidin S synthetase. Appl. Microbiol. Biotechnol. 24: 319–322.
Aharonowitz, Y. and A.L. Demain. 1979. Nitrogen nutrition and regulation of cephalosporin production inStreptomyces clavuligerus. Can. J. Microbiol. 25: 61–67.
Aharonowitz, Y., S. Mendelovitz, F. Kirenberg and V. Kuper. 1984. Regulatory mutants ofStreptomyces clavuligerus affected in free diaminopimelic acid content and antibiotic biosynthesis. J. Bacteriol. 157: 337–340.
Bradford, M. 1976. A rapid, sensitive method for the quantion of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 72: 248–254.
Flickinger, M.C. and D. Perlman. 1979. Application of oxygen-enriched aeration in the production of bacitracin byBacillus licheniformis. Antimicrob. Agents Chemother. 15: 282–293.
Friebel, T.E. and A.L. Demain. 1977. Oxygen-dependent inactivation of gramicidin S synthetase inBacilus brevis. J. Bacteriol. 130: 1010–1016.
Gaucher, G.M., K.S. Lam, J.W.D. GrootWassink, J. Neway and Y.M. Deo. 1981. The initiation and longevity of patulin biosynthesis. Dev. Ind. Microbiol. 22: 219–232.
Hanlon, G.W. and N.A. Hodges. 1981. Bacitracin and protease production in relation to sporulation during exponential growth ofBacillus licheniformis on poorly utilized carbon and nitrogen sources. J. Bacteriol. 147: 427–431.
Higgens, C.E., R.L. Hamill, T.H. Sands, M.M. Hoehn, N.E. Davis, R. Nagarajan and L.D. Boeck. 1974. The occurrence of deacetoxycephalosporin C in fungi andStreptomyces. J. Antibiot. 27: 298–300.
Hopwood, D.A., M.J. Bibb, K.F. Chater, T. Kieser, C.J. Bruton, H.M. Kieser, D.J. Lydiate, C.P. Smith, J.M. Ward and H. Schrempf. 1985. Preparation of organisms and phages. In: Genetic Manipulation of Streptomyces. A Laboratory Manual. The John Innes Foundation.
Jensen, S.E., B.K. Leskiw, L.C. Vining, Y. Aharonowitz and D.W.S. Westlake. 1986. Purification of isopenicillin N synthetase fromStreptimyces clavuligerus. Can. J. Microbiol. 32: 953–958.
Jensen, S.E., D.W.S. Westlake and S. Wolfe. 1982. Cyclization of δ(l-α-aminoadipyl)-l-cysteinyl-d-valine to penicillins by cell-free extracts ofStreptomyces clavuligerus. J. Antibiot. 35: 483–490.
Koplove, N.M. and C.L. Cooney. 1979. Enzyme production during transient growth: the reorganization of protein synthesis. In: Advances in the Biochemical Engineering of Immobilized Enzymes II, Vol. 12 (Ghose, T.K., A. Fiechter and N. Blakebrough, eds.), pp. 1–40, Springer-Verlag, Berlin.
Lilley, G., A.E. Clark and G.C. Lawrence. 1981. Control of the production of cephamycin C and thienamycin byStreptomyces cattleya NRRL 8057. J. Chem. Tech. Biotechnol. 31: 127–134.
Matteo, C.C., C.L. Cooney and A.L. Demain. 1976. Production of gramicidin S synthetases byBacillus brevis in continuous culture. J. Gen. Microbiol. 96: 415–422.
Revilla, G., F.R. Ramos, M.J. Lopez-Nieto, E. Alvarez and J.F. Martin. 1976. Glucose represses formation of δ(l-α-aminoadipyl)-l-cysteinyl-d-valine and isopenicillin N synthase but not penicillin acyltransferase inPenicillium chrysogenum. J. Bacteriol. 167: 947–952.
Sawada, Y., T. Konomi, N.A. Solomon and A.L. Demain. 1980. Increase in activity of β-lactam synthetases after growth ofCephalosporium acremonium with methionine or norleucine. FEMS Microbiol. Lett. 9: 281–284.
Seddon, B. and G.H. Fynn. 1973. Energetics of growth in a tyrothricin-producing strain ofBacillus brevis. J. Gen. Microbiol. 74: 305–314.
Turner, M.K., J.E. Farthing and S.J. Brewer. 1978. The oxygenation of [3-methyl-3H]-desacetoxycephalosporin C [7β-(5-d-aminoadipamido)-3-methylceph-3-em-4-carboxylic acid] to [3-hydroxymethyl-3H]desacetylcephalosporin C by 2-oxoglutarate-linked dioxygenases fromAcremonium chrysogenum andStreptomyces clavuligerus. Biochem. J. 173: 839–850.
Vandamme, E.J., D. Leyman, P. De Visscher, D. DeBuyser and G. Vansteenkiste. 1981. Effect of aeration and pH on gramicidin S production byBacillus brevis. J. Chem. Tech. Biotechnol. 31: 247–257.
Vardar, F. and M.D. Lilly. 1982. Effect of cycling D.O. concentrations on product formation in penicillin fermentations. Eur. J. Appl. Microbiol. Biotechnol. 14: 203–211.
Vu-Trong, K. and P.P. Gray. 1982. Stimulation of tylosin productivity resulting from cyclic feeding profiles in fed batch cultures. Biotechnol. Lett. 4: 725–728.
Vu-Trong, K. and P.P. Gray. 1984. Stimulation of enzymes involved in tylosin biosynthesis by cyclic feeding profiles in fed batch cultures. Biotechnol. Lett. 6: 435–440.
Wang, D.I.C. and R.C.L. Fewkes. 1977. Effect of operating and geometric parameters on the behaviour of non-newtonian, mycelial, antibiotic fermentations. Dev. Ind. Microbiol. 18: 39–56.
Zhang, J.-y., G. Banko, S. Wolfe and A.L. Demain. 1987. Methionine induction of ACV synthetase inCephalosporium acremonium. J. Ind. Microbiol. 2: 251–255.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rollins, M.J., Jensen, S.E. & Westlake, D.W.S. Effect of aeration on antibiotic production byStreptomyces clavuligerus . Journal of Industrial Microbiology 3, 357–364 (1988). https://doi.org/10.1007/BF01569557
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01569557