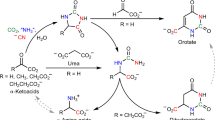
Abstract
The enteric lineage of prokaryotes (traditional enteric bacteria,Aeromonas, andAlteromonas) encompasses closely related genera that share many common character states of aromatic amino acid biosynthesis. For example, they uniformly employ the tightly regulated bifunctional P-protein (chorismate mutase: prephenate dehydratase) to forml-phenylalanine via phenylpyruvate. A second, unregulated pathway to phenylalanine, originally termed the overflow pathway inPseudomonas aeruginosa, consists of a monofunctional chorismate mutase (CM-F) and a cyclohexadienyl dehydratase. The evolution of the overflow pathway has been dynamic in the enteric lineage.Serratia marcescens, Erwinia herbicola, Erwinia amylovora, and several otherErwinia species possess an intact pathway.Salmonella, Klebsiella, andErwinia carotovora possess an incomplete overflow pathway, whileEscherichia, Proteus, Aeromonas, andAlteromonas lack it altogether.
Similar content being viewed by others
Literature Cited
Ahmad S, Jensen RA (1986) The evolutionary history of two bifunctional proteins that emerged in the purple bacteria. Trends Biochem Sci 11:108–112
Ahmad S, Jensen RA (1988) New prospects for deducing the evolutionary history of metabolic pathways in prokaryotes: aromatic biosynthesis as a case-in-point. Orig Life 18:41–57
Ahmad S, Jensen RA (1988) The phylogenetic origin of the bifunctional T-protein in the enteric lineage of bacteria. Mol Biol Evol (submitted)
Ahmad S, Jensen RA (1987) The prephenate dehydrogenase component of the bifunctional T-protein in enteric bacteria can utilizel-arogenate. FEBS Lett 216:133–139
Ahmad S, Johnson JL, Jensen RA (1981) The recent evolutionary origin of the phenylalanine-sensitive isozyme of 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase in the enteric lineage of bacteria. J Mol Evol 25:159–167
Baumann P, Gauthier MJ, Baumann L (1984) GenusAlteromonas. In: Krieg NR (ed) Bergey's manual of systematic bacteriology. Baltimore: Williams and Wilkins, pp.343–352
Berry A, Ahmad S, Liss A, Jensen RA (1987) Enzymological features of aromatic amino acid biosynthesis reflect the phylogeny of mycoplasmas. J Gen Microbiol 133:2147–2154
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Byng GS, Whitaker RJ, Shapiro CL, Jensen RA (1981) The aromatic amino acid pathway branches atl-arogenate inEuglena gracilis. Mol Cell Biol 1:426–438
Byng GS, Whitaker RJ, Jensen RA (1983) Evolution ofl-phenylalanine biosynthesis in rRNA homology group I ofPseudomonas. Arch Microbiol 136:163–168
Cotton RGH, Gibson F (1965) The biosynthesis of phenylalanine and tyrosine: enzymes converting chorismic acid into prephenic acid and their relationships to prephenate dehydratase and prephenate dehydrogenase. Biochim Biophys Acta 100:76–88
Crawford IP (1986) Gene fusions in the tryptophan pathway: tryptophan synthase and phosphoribosyl-anthranilate isomerase: indoleglycerol-phosphate synthase. In: Bisswanger H, Schmincke-Ott E (eds) Multifunctional proteins. New York: Wiley Interscience, pp 151–173
Davidson BE, Blackburn EH, Dopheide TAA (1972) Chorismate mutase-prephenate dehydratase fromEscherichia coli K-12 I. Purification, molecular weight, and amino acid composition. J Biol Chem 247:4441–4446
Dayan J, Sprinson DB (1970) Preparation of prephenic acid. Methods Enzymol 17A:559–561
Dayan J, Sprinson DB (1971) Enzyme alterations in tyrosine and phenylalanine auxotrophs ofSalmonella typhimurium, J Bacteriol 108:1174–1180
Delong EF, Baumann L, Bowditch RD, Baumann P (1984) Evolutionary relationships of superoxide dismutases and glutamine synthetases from marine species ofAlteromonas, Oceanospirillum, Pseudomonas andDeleya. Arch Microbiol 138:170–178
Fazel AM, Jensen RA (1980) Regulation of prephenate dehydratase in coryneform species of bacteria byl-phenylalanine and by remote effectors. Arch Biochem Biophys 200:165–176
Fiske MJ, Whitaker RJ, Jensen RA (1983) Hidden overflow pathway tol-phenylalanine inPseudomonas aeruginosa. J Bacteriol 154:623–631
Fox GE, Stackebrandt E, Hespell RB, Gibson J, Maniloff J, Dyer TA, Wolfe RS, Balch WE, Tanner RS, Magrum LJ, Zablen LB, Blakemore R, Gupta R, Bonen L, Lewis BJ, Stahl DA, Leuhrsen KR, Chen KN, Woese CR (1980) The phylogeny of prokaryotes. Science 209:457–463
Gibson F (1964) Chorismic acid. Purification and some chemical and physical studies. Biochem J 90:256–261
Jayaswal RK, Bressan RA, Handa AK (1984) Mutagenesis ofErwinia carotovora subsp.carotovora with bacteriophage Mu dl (Apr lac cts62): construction ofhis-lac gene fusions. J Bacteriol 158:764–766
Jensen RA (1985) Biochemical pathways in prokaryotes can be traced backward through evolutionary time. Mol Biol Evol 2:92–108
Jung E, Zamir LO, Jensen RA (1986) Chloroplasts of higher plants synthesizel-phenylalanine vial-arogenate. Proc Natl Acad Sci USA 83:7231–7235
Lindroth P, Mopper K (1979) High performance liquid chromatography determination of subpicomole amounts of amino acids by precolumn fluorescence derivatization witho-phthaldehyde. Anal Biochem 51:1667–1674
Miller TD, Schroth MN (1972) Monitoring the epiphytic population ofErwinia amylovora on pear with a selective medium. Phytopathology 62:1175–1182
Patel N, Pierson DL, Jensen RA (1977) Dual enzymatic routes tol-tyrosine andl-phenylalanine via pretyrosine inPseudomonas aeruginosa. J Biol Chem 252:5839–5846
Pierson DL, Jensen RA (1974) Metabolic interlock: control of an interconvertible prephenate dehydratase by hydrophobic amino acids inBacillus subtilis. J Mol Biol 90:563–579
Rebello JL, Jensen RA (1970) Metabolic interlock. The multi-metabolite control of prephenate dehydratase activity inBacillus subtilis. J Biol Chem 245:3738–3744
Stenmark SL, Pierson DL, Jensen RA, Glover GI (1974) Blue green bacteria synthesizel-tyrosine by the pretyrosine pathway. Nature 247:290–292
Verdonck L, Mergaert J, Rijckaert C, Kersters SK, Deley J (1987) GenusErwinia: numerical analysis of phenotypic features. Int J Syst Bacteriol 37:4–18
Whitaker RJ, Gaines CG, Jensen RA (1982) A multi-specific quintet of aromatic aminotransferases that overlap different biochemical pathways inPseudomonas aeruginosa. J Biol Chem 257:13550–13556
Whitaker RJ, Berry A, Byng GS, Fiske MJ, Jensen RA (1985) Clues fromXanthomonas campestris about the evolution of aromatic biosynthesis and its regulation. J Mol Evol 21:139–149
Winkler UK, Stuckman M (1979) Glycogen, hyaluronate and some other polysaccharides greatly enhance the formation of exolipase bySerratia marcescens. J Bacteriol 138:663–670
Woese CR, Weisburg WG, Paster BJ, Hahn CM, Tanner RS, Krieg NR, Koops HP, Harms H, Stackebrandt E (1984) The phylogeny of purple bacteria: the beta subdivision. Syst Appl Microbiol 5:327–336
Woese CR, Weisburg WG, Hahn CM, Paster BJ, Zablen LB, Lewis BJ, Macke TJ, Ludwig W, Stackebrandt E (1985) The phylogeny of purple bacteria: the gamma subdivision. Syst Appl Microbiol 6:25–33
Zamir LO, Jensen RA, Arison BH, Douglas AW, Albers-Schonberg G, Bowen JR (1980) Structure of arogenate (pretyrosine), an amino acid intermediate of aromatic biosynthesis. J Am Chem Soc 102:4499–4504
Zamir LO, Tiberio R, Fiske M, Berry A, Jensen RA (1985) Enzymatic and nonenzymatic dehydration reactions ofl-arogenate. Biochemistry 24:1607–1612
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ahmad, S., Jensen, R.A. Phylogenetic distribution of components of the overflow pathway tol-phenylalanine within the enteric lineage of bacteria. Current Microbiology 16, 295–302 (1988). https://doi.org/10.1007/BF01568535
Issue Date:
DOI: https://doi.org/10.1007/BF01568535