Abstract
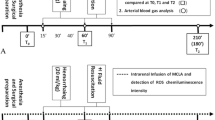
In this study we sought to define the role of oxygen-derived free radicals during ischemia and reperfusion in the production of acute damage to the gastric mucosa of baboons. The protective effect of the xanthine oxidase inhibitor, allopurinol, the superoxide scavenger, superoxide dismutase (SOD), and a long-acting SOD-albumin was determined. Mucosal damage was evaluated using light and scanning electron microscopy. Evidence for oxidative insult to the gastric mucosa was sought by measuring tissue concentrations of reduced (GSH) and oxidized (GSSG) glutathione. Gastric mucosal blood flow was estimated using the microsphere technique. A similar pattern of tissue damage was found at the end of ischemia in all three groups. Thirty minutes after reperfusion, severe mucosal damage (grade 3) increased only in the untreated control. In the two treated groups, grade 3 damage remained unchanged during reperfusion and a decrease in the percentage of moderate damage (grade 2) was seen. Both GSH and GSSG tissue concentrations were lower in the untreated controls as compared to the scavenger-treated groups, making it questionable whether GSH/GSSG tissue levels adequately reflect oxidative stress. We conclude that in our ischemiareperfusion model the generation of oxygen-derived free radicals produces mucosal damage and prevents the restitution of moderate mucosal damage during reperfusion. In ischemia, factors other than free radicals seem to be responsible for mucosal damage. The protective effect of allopurinol and SOD was not mediated by changes in gastric mucosal blood flow.
Similar content being viewed by others
References
Granger DN, Parks DA: Role of oxygen radicals in the pathogenesis of intestinal ischemia. Physiologist 26:159–164, 1983
Bounous G: Acute necrosis of the intestinal mucosa. Gastroenterology 82:1457–1467, 1982
Haglind E, Haglund U, Lundgren O, Romanus M, Schersten T: Graded intestinal vascular obstruction. 1. Description of an experimental shock model in the rat. Circ Shock 7:83–91, 1980
Parks DA, Grogaard B, Granger DN: Comparison of partial and complete occlusion models for studying intestinal ischemia. Surgery 92:896–902, 1982
Granger DN, Rutili G, McCord JM: Superoxide radicals in feline intestinal ischemia. Gastroenterology 81:22–29, 1981
Parks DA, Bulkley GB, Granger DN, Hamilton SR, McCord JM: Ischemic injury in the cat small intestine: Role of superoxide radicals. Gastroenterology 82:9–15, 1982
Schoenberg MH, Muhl E, Sellin D, Younes M, Schildberg FW, Haglund U: Posthypotensive generation of superoxide free radicals. Possible role in the pathogenesis of the intestinal mucosal damage. Acta Chir Scand 150:301–309, 1984
Itoh M, Guth PM: Role of oxygen-derived free radicals in hemorrhagic shock-induced gastric lesions in the rat. Gastroenterology 88:1162–1167, 1985
Perry MA, Wadhwa S, Parks DA, Pickard W, Granger DN: Role of oxygen radicals in ischemia-induced lesions in the cat stomach. Gastroenterology 90:362–367, 1986
Cleland LG, Bielicki J, Betts WH: Superoxide dismutase (SOD) conjugates with delayed clearance from plasma and body cavities. 12th International Biochemistry Congress. Perth, Australia, 1982, poster 005–14
Wong K, Cleland LG, Poznansky MJ: Enhanced anti-inflammatory effect and reduced immunogenicity of bovine liver superoxide dismutase by conjugation with homologous albumin. Agents Actions 10:231–238, 1980
Heymann MA, Payne BD, Hoffman JIE, Rudolph AM: Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis 20:55–79, 1977
Lacy ER, Ito S: Microscopic analysis of ethanol damage to rat gastric mucosa after treatment with a prostaglandin. Gastroenterology 83:619–625, 1982
Adams Jr, JD, Lauterburg BH, Mitchell JR: Plasma glutathione and glutathione disulfide in the rat: Regulation and response to oxidative stress. J Pharmacol Exp Ther 227:749–754, 1983
Crapo JD, McCord JM, Fridovich I: Preparation and assay of SOD. Methods Enzymol 53:382, 1978
Black BA, Morris GP, Wallace JL: Effects of acid on the basal lamina of the rat stomach and duodenum. Cell Pathol 50:109–118, 1985
Morris GP, Harding PL: Mechanisms of mucosal recovery from acute gastric damage: Roles of extracellular mucus and cell migration.In Mechanisms of Mucosal Protection in the Upper Gastrointestinal Tract. A Allen, G Flemstrom, A Garner, W Silen, LA Turnberg (eds). New York, Raven Press, 1984, pp 209–214
Morris GP, Wallace JL: The roles of ethanol and of acid in the production of gastric mucosal erosions in rats. Cell Pathol 38:23–38, 1981
Rutten MJ, Ito S: Morphology and electrophysiology of guinea pig gastric mucosal repair in vitro. Am J Physiol 244:G171-G182, 1983
Grisham MB, Hernandez LA, Granger DN: Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol 251:G567-G574, 1986
Werns SW, Shea MJ, Lucchesi BR: Free radicals in ischemic myocardial injury. J Free Radicals Biol Med 1:103–110, 1985
Remy MH, Poznansky MJ: Immunogenicity and antigenicity of soluble cross-linked enzyme/albumin polymers: Advantages for enzyme therapy. Lancet 2:68–70, 1978
Boyd SC, Sasame HA, Boyd MR: Gastric glutathione depletion and acute ulcerogenesis by diethylmaleate given subcutaneously to rats. Life Sci 28:2987–2992, 1981
Ghanayem BI, Boor PJ, Ahmed AE: Acrylonitril-induced gastric mucosal necrosis: Role of gastric glutathione. J Pharmacol Exp Ther 232:570–577, 1985
Keeling PL, Smith LL: Relevance of NADPH depletion and mixed disulfide formation in the rat lung to the mechanism of cell damage following paraquat administration. Biochem Pharmacol 31:3243–3249, 1982
Szabo S, Trier JS, Frankel PW: Sulfhydryl compounds may mediate gastric cytoprotection. Science 214:200–202, 1981
Brigelius R, Lenzen R, Sies H: Increases in hepatic mixed disulfide and glutathione disulflde levels elicited by paraquat. Biochem Pharmacol 31:1637–1641, 1982
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Von Ritter, C., Hinder, R.A., Oosthuizen, M.M.J. et al. Gastric mucosal lesions induced by hemorrhagic shock in baboons. Digest Dis Sci 33, 857–864 (1988). https://doi.org/10.1007/BF01550976
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01550976