Summary
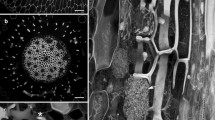
Cellulose microfibrils (MFs) were visualized on the inner surface of root cortex cell walls ofHyacinthus orientalis L. using a replica technique. Microfibril orientation was determined in radial longitudinal and transverse cell walls of the root tip, uncontracted, contracting, and fully contracted regions of the root. In longitudinal walls, the innermost MFs were ordered and parallel to one another and were oriented transversely, axially or obliquely, depending upon the developmental stage of the region. In transverse walls MFs in a single layer formed crisscross or ordered parallel arrays, depending upon the region. Parallel arrays were oriented either parallel, perpendicular, or oblique to the radius of the root. Inner walls of certain cells in the contracting region had MFs which appeared interrupted over their lengths. In general, these findings parallel earlier immunofluorescence and electron microscopic observations of changing cortical microtubule (MT) orientation accompanying root contraction. The major exception to MT-MF congruence occurred in cells of the actively contracting region. In middle and outer cell layers, MFs appeared short and partially obscured, while MTs in these cells occurred in conspicuous laterally aggregated strands parallel to one another over the length of the cells or were absent. This alteration in MF-MT parallelism may be related to the reorientation in cell growth occurring in the contractile zone or to the collapse of specific cells during the process of root contraction.
Similar content being viewed by others
Abbreviations
- MF:
-
microfibril
- MT:
-
microtubule
References
Cyr RJ, Lin B-L, Jernstedt JA (1988) Root contraction in hyacinth. II. Changes in tubulin levels, microtubule number and orientation associated with differential cell expansion. Planta 174: 446–452
Gunning BES, Hardham AR (1982) Microtubules. Annu Rev Plant Physiol 33: 651–698
Hardham AR (1982) Regulation of polarity in tissues and organs. In: Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 377–403
Hogetsu T (1986) Orientation of wall microfibril deposition in root cells ofPisum sativum L. var Alaska. Plant Cell Physiol 27: 947–951
—, Oshima Y (1986) Immunofluorescence microscopy of microtubule arrangement in root cells ofPisum sativum L. var Alaska. Plant Cell Physiol 27: 939–945
Jernstedt JA (1984) Root contraction in hyacinth. I. Effects of IAA on differential cell expansion. Amer J Bot 71: 1080–1089
Lang JM, Eisinger WR, Green PB (1982) Effects of ethylene on the orientation of microtubules and cellulose microfibrils of pea epicotyl cells with polylamellate cell walls. Protoplasma 110: 5–14
Lin B-L, Jernstedt JA (1987) Microtubule organization in root cortical cells ofHyacinthus orientalis. Protoplasma 141: 13–23
Lloyd CW (1984) Toward a dynamic helical model for the influence of microtubules on wall patterns in plants. Int Rev Cytol 86: 1–51
— (1987) The plant cytoskeleton: the impact of fluorescence microscopy. Annu Rev Plant Physiol 38: 119–139
—, Barlow PW (1982) The coordination of cell division and elongation: the role of the cytoskeleton. In: Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 103–228
Marchant HJ (1982) The establishment and maintenance of plant cell shape by microtubules. In: Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 295–319
Mita I, Shibaoka H (1984 a) Effects of root excision on swelling of leaf sheath cells and on the arrangement of cortical microtubules in onion seedlings. Plant Cell Physiol 25: 1521–1529
— — (1984 b) Effects of S-3307, an inhibitor of gibberellin biosynthesis, on swelling of leaf sheath cells and on the arrangement of cortical microtubules in onion seedlings. Plant Cell Physiol 25: 1531–1539
— — (1984 c) Gibberellin stabilizes microtubules in onion leaf sheath cells. Protoplasma 119: 100–109
Mueller SC, Brown RM Jr (1982) The control of cellulose microfibril deposition in the cell wall of higher plants. I. Can directed membrane flow orient cellulose microfibrils? Indirect evidence from freeze-fractured plasma membranes of maize and pine seedlings. Planta 154: 489–500
Preston RD (1988) Cellulose-microfibril-orienting mechanisms in plant cell walls. Planta 174: 67–74
Quader H (1986) Cellulose microfibril orientation inOocystis solitaria: proof that microtubules control the alignment of the terminal complexes. J Cell Sci 83: 223–234
Richmond PA (1983) Patterns of cellulose microfibril deposition and rearrangement inNitella: in vivo analysis by a birefringence index. J Appl Polym Sci: Appl Polym Symp 37: 107–122
—, Métraux J-P (1984) Cellulose synthesis inhibition, cell expansion, and patterns of cell wall deposition inNitella internodes. In: Dugger WM, Bartnicki-Garcia S (eds) Structure, function, and biosynthesis of plant cell walls. Proceedings of the Seventh Annual Symposium in Botany, University of California, Riverside, pp 475–476
Roberts IN, Lloyd CW, Roberts K (1985) Ethylene-induced microtubule reorientations: mediation by helical arrays. Planta 164: 439–447
Smith-Huerta NL, Jernstedt JA (1989) Root contraction in hyacinth III. Orientation of cortical microtubules visualized by immunofluorescence microscopy. Protoplasma 151: 1–10
Sterling C (1972) Mechanism of root contraction inGladiolus. Ann Bot 36 (ns): 599–603
Taiz L (1984) Plant cell expansion: regulation of cell wall mechanical properties. Annu Rev Plant Physiol 35: 585–657
Traas JA, Braat P, Derksen JW (1984) Changes in microtubule arrays during the differentiation of cortical root cells ofRaphanus sativus. Eur J Cell Biol 34: 229–238
Wick SM (1985) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. III. Transition between mitotic/cytokinetic and interphase microtubule arrays. Cell Biol Int Rep 9: 357–371
—, Seagull RW, Osborn M, Weber K, Gunning BES (1981) Immunofluorescence microscopy of organized microtubule arrays in structurally stabilized meristematic plant cells. J Cell Biol 89: 685–690
Wilson K, Honey JN (1966) Root contraction inHyacinthus orientalis. Ann Bot 30: 47–61
Zamski E, Ucko O, Koller D (1983) The mechanism of root contraction inGymnarrhena micranatha, a desert plant. New Phytol 95: 29–35
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Smith-Huerta, N.L., Jernstedt, J.A. Root contraction in hyacinth IV. Orientation of cellulose microfibrils in radial longitudinal and transverse cell walls. Protoplasma 154, 161–171 (1990). https://doi.org/10.1007/BF01539844
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01539844