Summary
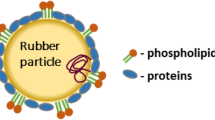
From centrifuged high-ammonia latex concentrate, a fraction which contains rubber particles of a narrow particle size distribution in a serum phase freed from any water-soluble non-rubber constituents was obtained. The electrophoretic mobility of the dialysed latex particles has been studied as a function of dry rubber content, surface pH and concentration of sodium chloride. The rubber particles were shown to have an amphoteric nature with an isoelectric point at pH 3.8. The electrokinetic study showed that the net negative charge on the rubber particle surface is derived from the carboxyl groups of the adsorbed proteins and the adsorbed long-chain fatty acids. This is in agreement with the infrared data of the rubber film. The surface charge density of the rubber particle at pH > 6 was found to be 1.43 ,µC cm−2. The stability of the latex is briefly discussed in terms of its surface charge and compared with that of synthetic latices.
Zusammenfassung
Durch Zentrifugieren von stark ammoniakhaltigen Latex-Konzentraten wurde eine Fraktion erhalten, welche Gummiteilchen mit einer engen Teilchengrößenverteilung in einem Serum enthielt, welches frei von wasserlöslichen Nichtgummibestandteilen war. Die elektrophoretische Beweglichkeit der dialysierten Latexteilchen wurde als Funktion des Gummigehaltes, des Oberflächen-pH-Wertes und der Natriumchloridkonzentration gemessen. Die Gummiteilchen verhalten sich amphoter mit einem isoelektrischen Punkt bei pH 3,8. Die Oberflächenladung rührt von Carboxylgruppen adsorbierter Proteine und adsorbierter langkettiger Fettsäuren her. Die Oberflächenladungsdichte bei pH > 6 wurde zu 1,43 ,µC σ cm−2 bestimmt. Die Stabilität wird im Zusammenhang mit der Ladungsdichte diskutiert und mit der von synthetischen Latices verglichen.
Similar content being viewed by others
References
Weber, C. O., The Chemistry of Indian Rubber (London 1903).
Belgrave, W. N. C., Malayan Agric. J.11, 348 (1923);13, 369 (1925).
Kemp I., D. F. Twiss, Trans. Faraday Soc.32, 890 (1936).
Bondy, C., H. Freundlich, Rubb. Chem. Technol.11, 601 (1938).
Kemp, A. R., W. G. Straitiff, J. Phys. Chem.44, 788 (1940).
Archer, B. L., B. C. Sekhar, Biochem. J.61, 503 (1955).
Ho, C. C., A. Subramaniam, W. M. Yong, Proc. International Rubber Conference 1975, Vol. 2, p. 441 (Kuala Lumpur 1976).
Archer, B. L., D. Barnard, E. G. Cockbain, P. B. Dickenson, A. I. McMullen, In: L. Bateman (Ed.), The Chemistry and Physics of Rubberlike Substances, p. 41 (London 1963).
van der Tempel, M., Trans. I. R. I.28, 303 (1952).
Salomon, G., A. Chr. van der Schee, J. Polymer Sci.14, 181 (1954).
Gregg, E. C., J. H. Macey, Rubb. Chem. Technol.46, 47 (1973).
Bowler, W. W., Ind. Eng- Chem.45, 1790 (1953).
Ottewill, R. H., J. N. Shaw, J. Electroanal. Chem.37, 133 (1972).
Wiersema, P. H., A. Loeb, J. Th. G. Overbeek. J. Colloid and Interface Sci.22, 78 (1966).
Hartley, G. S., J. W. Roe, Trans. Faraday Soc,36, 101 (1940).
Ottewill, R. H., J. N. Shaw, Kolloid-Z. u. Z. Polymers218, 34 (1967).
Ottewill, R. H., D. J. Wilkins, Trans. Faraday Soc.58, 608 (1962).
Bangham, A. D., B. A. Pethica, G. V. F. Seaman, Biochem. J.69, 12 (1958).
Abramson, H. A., Electrophoresis of Proteins (New York 1964).
Mehrishi, J. N., G. V. F. Seaman, Trans. Faraday Soc.64, 3152 (1968).
Rideal, E. K., D. M. Adams, In:J. F. Danielli, K. G. A. Pankhurst, A. C. Riddiford (Ed.), Surface Phenomena in Chemistry and Biology, p. 309 (London 1958).
Ho, C. C., unpublished work.
Altman, R. F. A., Rubb. Chem. Technol.19, 788 (1946).
Brash, J. L., D. J. Lyman, In:M. L. Hair (Ed.), The Chemistry of Biosurfaces, Vol. 1, p. 177 (New York 1971).
Force, C. G., A. S. Wilson, J. Colloid Interface Sci.32, 477 (1970).
Stone-Masui, J., A. Watillon, J. Colloid Interface Sci.52, 479 (1975).
Force, C. G., E. Matijevic, J. P. Kratohvil, Kolloid-Z. u. Z. Polymers223, 31 (1968).
Douglas, H. W., Trans. Faraday Soc.55, 850 (1959).
Gittens, G. J., A. M. James, Biochim. Biophys. Acta.66, 237 (1963);66, 250 (1963).
Bangham, A. D., B. A. Pethica, Proc. Roy. Phys. Soc. Edinburgh28, 43 (1960).
Goodwin, J. W., J. Hearn, C. C. Ho, R. H. Ottewill, Brit. Polymer J.5, 347 (1973).
van den Hul, H. J., J. W. Vanderhoff, In:R. M. Fitch (Ed. ), Polymer Colloids, p. 1 (New York 1971).
Author information
Authors and Affiliations
Additional information
With 7 figures and 1 table
Rights and permissions
About this article
Cite this article
Ho, C.C., Ng, W.L. Surface study on the rubber particles in pretreated Hevea latex system. Colloid & Polymer Sci 257, 406–412 (1979). https://doi.org/10.1007/BF01521577
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01521577