Summary
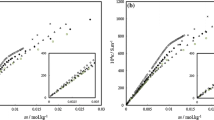
By means of light scattering techniques, the micellar weight of methoxy-polyoxyethylene decyl ether (MPd-12) and that of sodium dodecyl sulfate (SDS) have been measured as a function of temperature. The effects of the solubilized n-decane and n-decanol on the micellar weight have also been studied at different temperatures. From the data it has been concluded: 1) that the micellar weight of MPd-12 increases with elevation of temperature, whereas the micellar weight of SDS decreases with temperature; 2) that the apparent second virial coefficient in the aqueous solution of MPd-12 decreases with temperature and that in 0.1 m. NaCl solution of SDS increases with temperature; 3) that in the aqueous solution of MPd-12 containing solubilized n-decane or n-decanol, the micellar weight increases with temperature in both cases, but the patterns of increase are different from each other.
Zusammenfassung
Mittels Lichtstreuung wurden die Mizellgewichte von Methoxypolyoxyäthylendecyläther (MPd-12) und von Natriumdodecylsulfat (SDS) als Punktion der Temperatur gemessen. Die Effekte des solubilisierten n-Dekans und n-Dekanols auf das Mizellgewicht wurden bei verschiedener Temperatur untersucht. Aus den Ergebnissen ist zu schließen:
-
1.
Das Mizellgewicht von MPd-12 wächst mit Temperaturerhöhung, während das Mizellgewicht von SDS absinkt.
-
2.
Der scheinbare zweite Virialkoeffizient des MPd-12 in wäßriger Lösung nimmt mit der Temperatur ab und der von SDS in 0,1 molarer Lösung steigt mit der Temperatur.
-
3.
Für MPd-12-Lösung in Wasser, die solubilisierendes Dekan oder Dekanol enthält, wächst das Mizellgewicht in beiden Fällen mit der Temperatur, aber die Art des Anstiegs ist unterschiedlich.
Similar content being viewed by others
References
Debye, P., J. Phys. Chem.50, 1 (1949).
Debye, P., Ann. N. Y. Acad. Sci.51, 575 (1949).
Phillips, J. N. andK. J. Mysels, J. Phys. Chem.59, 325 (1955).
Kushner, L. M. andW. D. Hubbard, J. Phys. Chem.58, 1163 (1954).
Kushner, L. M., W. D. Hubbard andA. S. Doan, J. Phys. Chem.61, 371 (1957).
Nakagawa, T., K. Kuriyama andH. Inoue, J. Colloid Sci.15, 268 (1960).
Nakagawa, T. andK. Tori, Kolloid-Z.168, 132 (1960).
Kuriyama, K., H. Inoue andT. Nakagawa, Ann. Report, Shionogi Research Laboratory9, 1061 (1959).
Nakagawa, T. andI. Nakata, Kōgyō Kagaku Zasshi59, 710, 1154 (1956), cf. C. A.52, 4418, 14199 (1958).
McBain, W. andS. S. Marsden, jr., J. Chem. Phys.15, 211 (1947).
Layton, L. H. andU. P. Strauss, J. Colloid Sci.9, 149 (1954).
Mysels, K. J., J. Phys. Chem.58, 303 (1954).
Philippoff, W., Disc. Faraday Soc.11, 96 (1951).
Hutchinson, E., J. Colloid Sci.9, 191 (1954).
Mattoon, R. W., R. S. Stearns andW. D. Harkins, J. Chem. Phys.16, 644 (1948).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kuriyama, K. Temperature dependence of micellar weight of non-ionic surfactant in the presence of various additives. Kolloid-Z.u.Z.Polymere 180, 55–64 (1962). https://doi.org/10.1007/BF01499484
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01499484