Abstract
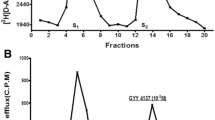
The effects of spontaneous and evoked [3H]taurine release from a P2 fraction prepared from rat retinas were studied. The P2 fraction was preloaded with [3H]taurine under conditions of high-affinity uptake and then examined for [3H]taurine efflux utilizing superfusion techniques. Exposure of the P2 fraction to high K+ (56 mM) evoked a Ca2+-independent release of [3H]taurine. Li+ (56 mM) and veratridine (100 μM) had significantly less effect (8–15% and 15–30%, respectively) on releasing [3H]taurine compared to the K+-evoked release. 4-Aminopyridine (1 mM) had no effect on the release of [3H]taurine. The spontaneous release of [3H]taurine was also Ca2+-independent. When Na+ was omitted from the incubation medium K+-evoked [3H]taurine release was inhibited by approximately 40% at the first 5 minute depolarization period but was not affected at a second subsequent 5 minute depolarization period. The spontaneous release of [3H]taurine was inhibited by 60% in the absence of Na+. Substitution of Br− for Cl− had no effect on the release of either spontaneous or K+-evoked [3H]taurine release. However, substitution of the Cl− with acetate, isethionate, or gluconate decreased K+-evoked [3H]taurine release. Addition of taurine to the superfusion medium (homoexchange) resulted in no significant increase in [3H]taurine efflux. The taurine-transport inhibitor guanidinoethanesulfonic acid increased the spontaneous release of [3H]taurine by approximately 40%. These results suggest that the taurine release of [3H]taurine is not simply a reversal of the carrier-mediated uptake system. It also appears that taurine is not released from vesicles within the synaptosomes but does not rule out the possibility that taurine is a neurotransmitter. The data involving chloride substitution with permeant and impermeant anions support the concept that the major portion of [3H]taurine release is due to an osmoregulatory action of taurine while depolarization accounts for only a small portion of [3H]taurine release.
Similar content being viewed by others
References
Jacobsen, J. G., and Smith, L. H., Jr. 1968. Biochemistry and physiology of taurine and taurine derivatives. Physiol. Rev. 48:424–511.
Voaden, M. J., Oraedu, A. C. I., Marshall, J., and Lake, N. 1981. Taurine in the retina. Pages 145–160,in Schaffer, S. W., Baskin, S. I. and Kocsis, J. J. (eds.), The Effects of Taurine on Excitable Tissues, Spectrum Publications, New York.
Hayes, K. C., Carey, R. E., and Schmidt, S. Y. 1975. Retinal degeneration associated with taurine deficiency in the cat. Science 188:949–951.
Sturman, J. A. 1983. Taurine in nutrition research. Pages 281–295,in Kuriyama, K., Huxtable, R. J., and Iwata, H. (eds.), Sulfur Amino Acids: Biochemical and Clinical Aspects, Alan R. Liss, New York.
Pasantes-Morales, H., Quesada, O., Cárabez, A., and Huxtable, R. J. 1983. Effects of the taurine transport antagonist, guanidinoethane sulfonate, and β-alanine on the morphology of rat retina. J. Neurosci. Res. 9:135–143.
Cocker, S. E., and Lake, N. 1987. Electroretinographic alterations and their reversal in rats treated with guanidinoethyl sulfonate, a taurine depletor. Exp. Eye Res. 45:977–987.
Neuringer, M., Imaki, H., Sturman, J. A., Moretz, R., and Wisniewski, H. M. 1987. Abnormal visual acuity and retinal morphology in rhesus monkeys fed a taurine-free diet during the first three postnatal months. Pages 125–134,in Huxtable, R. J., Franconi, F., and Giotti, A. (eds.), The Biology of Taurine: Methods and Mechanisms, Plenum Press, New York.
Geggel, H. S., Ament, M. E., Heckenlively, J. R., Mattin, D. A., and Kopple, J. D. 1985. Nutritional requirement for taurine in patients receiving long-term parenteral nutrition. New Eng. J. Med. 312:142–146.
Lombardini, J. B. 1991. Taurine: retinal function. Br. Res. Rev. 16:151–169.
Taber, K. H., Lin, C.-T., Liu, J.-W., Thalmann, R. H., and Wu, J.-Y. 1986. Taurine in hippocampus: localization and postsynaptic action. Brain Res. 386:113–121.
Hanretta, A. T., and Lombardini, J. B. 1987. Is taurine a hypothalamic neurotransmitter?: a model of the differential uptake and compartmentalization of taurine by neuronal and glial cell particles from the rat hypothalamus. Brain Res. Rev. 12:167–201.
Lombardini, J. B. 1985. Taurine effects on the transition temperature in Arrhenius plots of ATP-dependent calcium ion uptake in rat retinal membrane preparations. Biochem. Pharmacol. 34:3741–3745.
Huxtable, R. J., and Sebring, L. A. 1983. Cardiovascular actions of taurine. Pages 5–37,in Kuriyama, K., Huxtable, R. J., and Iwata, H. (eds.), Sulfur Amino Acids: Biochemical and Clinical Aspects, Alan R. Liss, New York.
Huxtable, R. J., and Sebring, L. A. 1989. Taurine and the heart: the phospholipid connection. Pages 31–42,in Iwata, H., Lombardini, J. B., and Segawa, T. (eds.), Taurine and the Heart, Kluwer Academic Publishers, New York.
Kuo, C.-H., and Miki, N. 1980. Stimulatory effect of taurine on Ca-uptake by disc membranes from photoreceptor cell outer segments. Biochem. Biophys. Res. Commun. 94:646–651.
Pasantes-Morales, H. and Ordóñez, A. 1982. Taurine activation of a bicarbonate-dependent ATP-supported calcium uptake in frog rod outer segments. Neurochem. Res. 7:317–328.
Liebowitz, S. M., Lombardini, J. B., and Salva, P. S. 1987. Cyclic taurine analogs: synthesis and effects on ATP-dependent Ca++ uptake in rat retina. Biochem. Pharmacol. 36:2109–2114.
Lombardini, J. B., Liebowitz, S. M., and Chou, T.-C. 1989. Analogues of taurine as stimulators and inhibitors of ATP-dependent calcium ion uptake in the rat retina: combination kinetics, Mol. Pharmacol. 36:256–264.
Lombardini, J. B. 1985. Effects of taurine on calcium ion uptake and protein phosphorylation in rat retinal membrane preparations. J. Neurochem. 45:268–275.
Lombardini, J. B. 1992. Effect of taurine on the phosphorylation of specific proteins in subcellular fractions of the rat retina. Neurochem. Res. 17:821–824.
Liebowitz, S. M., Lombardini, J. B., and Allen, C. I. 1988. Effects of aminocycloalkanesulfonic acid analogs of taurine on ATP-dependent calcium ion uptake and protein phosphorylation. Biochem. Pharmacol. 37:1303–1309.
Liebowitz, S. M., Lombardini, J. B., and Allen, C. I. 1989. Sulfone analogues of taurine as modifiers of calcium uptake and protein phosphorylation in rat retina. Biochem. Pharmacol. 38:399–406.
Li, Y.-P., and Lombardini, J. B. 1990. Taurine inhibits the phosphorylation of two endogenous proteins (Mr ∼ 140 and ∼ 20K) in subcellular preparations of rat cortex. Neurochem. Int. 17:389–399.
Li, Y.-P., and Lombardini, J. B. 1991. Taurine inhibits protein kinase C-catalyzed phosphorylation of specific proteins in a rat cortical P2 fraction. J. Neurochem. 56:1747–1753.
Li, Y.-P., and Lombardini, J. B. 1991. Inhibition by taurine of the phosphorylation of specific synaptosomal proteins in the rat cortex: Effects of taurine on the stimulation of calcium uptake in mitochondria and inhibition of phosphoinositide turnover. Brain Res. 553:89–96.
Barchas, J. D., Akil, H., Elliott, G. R., Holman, R. B., and Watson, S. J. 1978. Behavioral neurochemistry: neuroregulations and behavioral states. Science 200:964–973.
López-Colomé, A. M., and Pasantes-Morales, H. 1980. Taurine interactions with chick retinal membranes. J. Neurochem. 34:1047–1052.
Lombardini, J. B., and Prien, S. D. 1983. Taurine binding by rat retinal membranes. Exp. Eye Res. 37:239–250.
Schmidt, S. Y., and Berson, E. L. 1978. Taurine uptake in isolated retinas of normal rats and rats with hereditary retinal degeneration. Exp. Eye Res. 27:191–198.
Salceda, R. 1980. High-affinity taurine uptake in developing retina. Neurochem. Res. 5:561–572.
Redburn, D. A. and Thomas, T. N. 1979. Isolation of synaptosomal fractions from rabbit retina. J. Neurosci. Methods 1:235–242.
Hanretta, A. T., and Lombardini, J. B. 1986. Properties of spontaneous and evoked release of taurine from hypothalamic crude P2 synaptosomal preparations. Brain Res. 378:205–215.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275.
Levi, G., Gallo, V., and Raiteri, M. 1980. A re-evaluation of veratridine as a tool for studying the depolarization-induced release of neurotransmitters from nerve endings. Neurochem. Res. 5:281–295.
Saransaari, P. and Oja, S. S. 1992. Release of GABA and taurine from brain slices. Prog. Neurobiol. 38:455–482.
Lombardini, J. B. 1992. Potassium-stimulated release of taurine in a crude retinal preparation obtained from the rat is calcium independent. Pages 399–404,in Lombardini, J. B., Schaffer, S. W., and Azuma, J. (eds.), Taurine: Nutritional Value and Mechanisms of Action, Plenum Press, New York.
Shain, W., Connor, J. A., Madelian, V., and Martin, D. L. 1989. Spontaneous and beta-adrenergic receptor-mediated taurine release from astroglial cells are independent of manipulations of intracellular calcium. J. Neurosci. 9:2306–2312.
Martin, D. L., Madelian, V., and Shain, W. 1989. Spontaneous and beta-adrenergic receptor-mediated taurine release from astroglial cells do not require extracellular calcium. J. Neurosci. Res. 23:191–197.
Shain, W., Madelian, V., Waniewski, R. A., and Martin, D. L. 1990. Characteristics of taurine release from astroglial cells. Pages 299–306,in Pasantes-Morales, H., Martin, D. L., Shain, W., and Martin del Rio, R. (eds.) Taurine: Functional Neurochemistry, Physiology, and Cardiology, Wiley-Liss, New York.
Schousboe, A., and Pasantes-Morales, H. 1989. Potassium-stimulated release of [3H]taurine from cultured GABAergic and glutamatergic neurons. J. Neurochem. 53:1309–1315.
Holopainen, I., Kontro, P., and Oja, S. S. 1989. Release of taurine from cultured cerebellar granule cells and astrocytes: Co-release with glutamate. Neurosci. 29:425–432.
Pasantes-Morales, H., Dominguez, L., Montenegro, J., and Morán, J. 1988. A chloride-dependent component of the release of labeled GABA and taurine from the chick retina. Brain Res. 459:120–130.
Morán, J., Hurtado, S., and Pasantes-Morales, H. 1991. Similar properties of taurine release induced by potassium and hyposmolarity in the rat retina. Exp. Eye. Res. 53:347–352.
Kontro, P. and Oja, S. S. 1987. Taurine and GABA release from mouse cerebral cortex slices: Potassium stimulation releases more taurine than GABA from developing brain. Dev. Br. Res. 37:277–291.
Oja, S. S., and Kontro, P. 1989. Release of endogenous taurine and γ-aminobutyric acid from brain slices from the adult and developing mouse. J. Neurochem. 52:1018–1024.
Philibert, R. A., Rogers, K. L., Allen, A. J., and Dutton, G. R. 1988. Dose-dependent, K+-stimulated efflux of endogenous taurine from primary astrocyte cultures is Ca2+ dependent. J. Neurochem. 51:122–126.
Philibert, R. A., Rogers, K. L., and Dutton, G. R. 1989. K+-evoked taurine efflux from cerebellar astrocytes: On the roles of Ca2+ and Na+. Neurochem. Res. 14:43–48.
Philibert, R. A., Rogers, K. L., and Dutton, G. R. 1989. Stimulus-coupled taurine efflux from cerebellar neuronal cultures: On the roles of Ca++ and Na+. J. Neurosci. Res. 22:167–171.
Holopainen, I., Kontro, P., and Oja, S. S. 1985. Release of preloaded taurine and hypotaurine from astrocytes in primary culture: Stimulation by calcium-free media. Neurochem. Res. 10:123–131.
Oja, S. S., and Kontro, P. 1987. Cation effects on taurine release from brain slices: Comparison to GABA. J. Neurosci. Res. 17:302–311.
Solís, J. M., Herranz, A. S., Herreras, O., Muñoz, M. D., Martín del Ró, R., and Lerma, J. 1986. Variation of potassium ion concentrations in the rat hippocampus specifically affects extracellular taurine levels. Neurosci. Lett. 66:263–268.
Tigges, G. A., Philibert, R. A., and Dutton, G. R. 1990. K+-and temperature-evoked taurine efflux from hypothalamic astrocytes. Neuroscience Letters 119:23–26.
Kürzinger, K., and Hamprecht, B. 1981. Na+-dependent uptake and release of taurine by neuroblastoma × glioma hybrid cells. J. Neurochem. 37:956–967.
Korpi, E. R., and Oja, S. S. 1983. Characteristics of taurine release from cerebral cortex slices induced by sodium-deficient media. Brain Res. 289:197–204.
Oja, S. S., Korpi, E. R., Holopainen, I., and Kontro, P. 1985. Mechanisms of stimulated taurine release from nervous tissue. Pages 237–247,in Oja, S. S., Ahtree, L., Kontro, P., and Paasonen, M. K. (eds.), Taurine: Biological Actions and Clinical Perspectives, Alan R. Liss, New York.
Kontro, P., and Oja, S. S. 1989. Release of taurine and GABA from cerebellar slices from developing and adult mice. Neurosci. 29:413–423.
Domínguez, L., Montenegro, J., and Pasantes-Morales, H. 1989. A volume-dependent, chloride-sensitive component of taurine release stimulated by potassium from retina. J. Neurosci. Res. 22:356–361.
Sánchez Olea, R., and Pasantes-Morales, H. 1990. Chloride dependence of the K+-stimulated release of taurine from synaptosomes. Neurochem. Res. 15:535–540.
Pasantes-Morales, H., Morán, J., and Schousboe, A. 1990. Taurine release associated to cell swelling in the nervous system. Pages 369–376,in Pasantes-Morales, H., Martin, D. L., Shain, W., and Martín del Río, R. (eds.), Taurine: Functional Neurochemistry, Physiology, and Cardiology, Wiley-Liss, New York.
Solís, J. M., Herranz, A. S., Herreras, O., Lerma, J., and Martín del Río, R. 1988. Low chloride-dependent release of taurine by a furosemide-sensitive process in the in vivo rat hippocampus. Neurosci. 24:885–891.
Solís, J. M., Herranz, A. S., Herraras, O., Menéndez, N., and Martín del Río, R. 1990. Weak organic acids induce taurine release through an osmotic-sensitive process in in vivo rat hippocampus. J. Neurosci. Res. 26:159–167.
Martín del Río, R., Herranz, A. S., Herraras, O., Menéndez, N., and Solís, J. M. 1990. Possible osmoregulatory role of taurine in the cellular swelling evoked by weak organic acids in the rat hippocampus. Pages 357–368,in Pasantes-Morales, H., Martin, D. L., Shain, W., and Martín del Río, R. (eds.), Taurine: Functional Neurochemistry, Physiology, and Cardiology, Wiley-Liss, New York.
Huxtable, R. J., Laird, H. E., and Lippincott, S. E. 1979. The transport of taurine in the heart and the rapid depletion of tissue taurine content by guanidinoethyl sulfonate. J. Pharmacol. Exp. Ther. 211:465–471.
Adam-Vizi, V., Banay-Schwartz, M., Wajda, I., and Lajtha, A. 1987. Depolarization of brain cortex slices and synaptosomes by lithium. Determination of K+-equilibrium potential in cortex slices. Brain Res. 410:257–263.
Blaustein, M. P. 1975. Effects of potassium, veratridine, and scorpion venom on calcium accumulation and transmitter release by nerve terminals in vitro. J. Physiol. (London). 47:617–655.
Bullock, T. H., Orkland, R., and Grinnell, A. 1977. Introduction to Nervous Systems. Pages 129–176. W. H. Freeman, San Francisco.
Varon, S. S., and Somjen, G. G. 1979. Neuron-glial interactions. Neurosci. Res. Prog. Bull. 17:7–239.
Catterall, W. 1980. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annual Review of Pharmacology and Toxicology 20:15–43.
Reiser, G., and Hamprecht, B. 1983. Sodium-channels in nonexcitable glioma cells, shown by the influence of veratridine, scorpion toxin and tetrodotoxin on membrane potential and on ion transport. Pflugers Arch. 397:260–264.
Sarthy, P. V. 1983. Release of [3H]γ-aminobutyric acid from glial (Müller) cells of the rat retina; effects of K+, veratridine, and ethylenediamine. J. Neurosci. 3:2494–2503.
Bowman, C. L., Kimelbeg, H. K., Frangakis, M. V., Berwald-Netter, Y., and Edwards, C. 1984. Astrocytes in primary culture have chemically activated sodium channels. J. Neurosci. 4:1527–1534.
Philibert, R. A., and Dutton, G. R. 1989. Dihydropyridines modulate K+-evoked amino acid and adenosine release from cerebellar neuronal cultures. Neurosci. Lett. 102:97–102.
Yazulla, S. 1983. Stimulation of GABA release from retinal horizontal cells by potassium and acidic amino acid agonists. Brain Research 275:61–74.
Schwartz, E. A. 1987. Depolarization without calcium can release γ-aminobutyric acid from a retinal neuron. Science 238:350–355.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lombardini, J.B. Spontaneous and evoked release of [3H]taurine from a P2 subcellular fraction of the rat retina. Neurochem Res 18, 193–202 (1993). https://doi.org/10.1007/BF01474684
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01474684