Abstract
Heterochromatin behaviour and structural alterations in chromosomes of cells derived from callus culture ofAllium fistulosum have been studied.
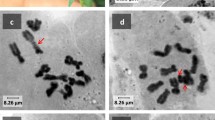
The diploid chromosome complement ofAllium fistulosum consists of 16 chromosomes with significant amount of heterochromatin mainly of telomeric nature. In eight collections of callus cells analysed, a high rate of numerical and structural chromosome abnormalities was observed. After 12 months in culture about 20% of metaphase chromosomes possessed distinct signs of mutational events.
C-banded preparations revealed that many structural alterations involved regions of heterochromatin. Interchromosomal connections and chromatid fusions occurred at telomeric heterochromatin segments. Also formation of the end-to-end associations and polycentric chromosomes often took place without visible loss of telomeric heterochromatin.
Similar content being viewed by others
References
Badr, A. & T.T. Elkington, 1977. Variation of Giemsa C-band and fluorochrome banded karyotypes, and relationships inAllium subgen.Molium. Pl. Syst. Evol. 128: 23–35.
Blackburn, E.H., 1990. Telomeres and their synthesis. Science 249: 489–490.
Blackburn, E.H., 1991. Structure and function of telomeres. Nature 350: 569–572.
Fiskesjö, G., 1975. Chromosomal relationships between three species ofAllium as revealed by C-banding. Hereditas 81: 23–32.
Geber, G. & D. Schweizer, 1988. Cytochemical heterochromatin differentiation inSinapsis alba (Cruciferae) using a simple air drying technique for producing chromosome spreads. Pl. Syst. Evol. 158: 97–106.
Godin, D. & S. Stack, 1976. Homologous and non-homologous chromosome associations by interchromosomal chromatin connectives inOrnithogalum virens. Chromosoma 57: 309–318.
Gustafson, J.P., A.J. Łukaszewski & M.D. Bennett, 1983. Somatic deletion and redistribution of telomeric heterochromatin in the genusSecale andTriticale. Chromosoma 88: 293–298.
Harley, C.B., A.B. Futcher & C.W. Greider, 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345: 458–460.
Holmquist, G. & M. Dancis, 1980. A general model of karyotype evolution. Genetica 52/53: 151–163.
Joachimiak, A., L. Przywara, T. Ilnicki & A. Kowalska, 1993. Megachromosomes in tissue culture ofAllium. Genetica 90: 35–40.
Johnson, S.S., R.L. Phillips, H.W. Rines, 1987a. Meiotic behaviour in progeny of tissue culture regenerated oat plants (Avena sativa L.) carrying near-telocentric chromosomes. Genome 29: 431–438.
Johnson, S.S., R.L. Phillips & H.W. Rines, 1987b. Possible role of heterochromatin in chromosome breakage induced by the tissue culture in oats (Avena sativa L.). Genome 29: 439–446.
Lapitan, N.L.V., R.G. Sears & B.S. Gill, 1984. Translocations and other karyotypic structural changes in wheat x rye hybrids regenerated from tissue culture. Theor. Appl. Genet. 68: 547–554.
Lee, M. & R.L. Phillips, 1988. The chromosomal basis of somaclonal variation. Ann. Rev. Plant Physiol. Plant Mol. Biol. 39: 413–437.
Lima-de-Faria, A., 1969. DNA replication and gene amplification in heterochromatin. In: Handbook of molecular cytology, A. Lima-de-Faria ed. North Holland Publishers Co., Amsterdam and London. pp. 277–325.
Marks, G.E., 1977. The nature of centromeric dots inNigella chromosomes. Chromosoma 62: 369–373.
Mayfield, J.E. & J.R. Ellison, 1975. The organization of interphase chromatin inDrosophilidae. The self adhesion of chromatin containing the same DNA sequence. Chromosoma 52: 37–48.
McClintock, B., 1978. Mechanisms that rapidly reorganize the genome. Stadler Genet. Symp. 10: 25–48.
McClintock, B., 1941. The stability of broken ends of chromosomes inZea mays. Genetics 26: 234–282.
McCoy, T.J., R.L. Phillips & H.W. Rines, 1982. Cytogenetic analysis of plants regenerated from oat (Avena sativa) tissue cultures; high frequency of partial chromosome loss. Can. J. Genet. Cytol. 24: 37–50.
Murashige, T. & Skoog, F., 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Pl. 15, 473–497.
Rhoades, M.M. & E. Dempsey, 1972. On the mechanism of chromatin loss induced by the B chromosome of maize. Genetics 71: 73–96.
Sacristan, M.D., 1971. Karyotypic changes in callus cultures from haploid and diploid plants ofCrepis capillaris (L.) Wallr. Chromosoma 33: 273–283.
Schwarzacher, T., P. Ambros & D. Schweizer, 1980. Application of Giemsa banding to orchid karyotype analysis. Pl. Syst. Evol. 134: 293–297.
Stelly, D.M., D.W. Altman, R.J. Kohel, T.S. Rangan & E. Commiskey, 1989. Cytogenetic abnormalities of cotton somaclones from callus cultures. Genome 32: 762–770.
Vig, B.K. & B.T. Richards, 1992. Formation of primary constriction and heterochromatin in mouse does not require minor satellite DNA. Exp. Cell Res. 201: 292–298.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Joachimiak, A., Ilnicki, T., Kowalska, A. et al. Chromosome alterations in tissue culture cells ofAllium fistulosum . Genetica 96, 191–198 (1995). https://doi.org/10.1007/BF01439572
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01439572