Abstract
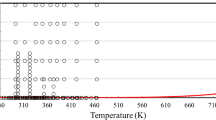
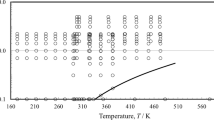
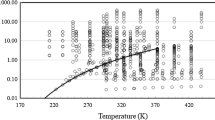
A new representation of the viscosity of ethane is presented. The representative equations are based upon a body of experimental data that have been critically assessed for internal consistency and for agreement with theory in the zero-density limit, vapor phase, and critical region. The representation extends over the temperature range from 100 K to the critical temperature in the liquid phase and from 200 K to the critical temperature in the vapor phase. In the supercritical region, the temperature range extends to 1000 K for pressures up to 2 MPa and to 500 K for pressures up to 60 MPa. The ascribed accuracy of the representation varies according to the thermodynamic state from ±0.5 % for the viscosity of the dilute gas near room temperature to ±3.0% for the viscosity at high pressures and temperatures. Tables of the viscosity, generated by the relevant equations, at selected temperatures and pressures and along the saturation line, are also provided.
Similar content being viewed by others
References
M. J. Assael, C. A. Nieto de Castro, and W. A. Wakeham,Chem. Por. 78:16.1 (1978).
V. Vesovic, W. A. Wakeham, G. A. Olchowy, J. V. Sengers, J. T. R. Watson, and J. Millat,J. Phys. Chem. Ref. Data 19:763 (1990).
I. F. Golubev,The Viscosity of Gases and Gas Mixtures (Israel Program for Scientific Translations, Jerusalem, 1970).
T. Makita, Y. Tanaka, and A. Nagashima,Rev. Phys. Chem. (Jap.) 44:98 (1974).
H. J. M. Hanley, K. E. Gubbins, and S. Murad,J. Phys. Chem. Ref. Data 6:1167 (1977).
A. A. Tarzimanov, V. E. Lyusternik, and V. A. Arslanov,Viscosity of Gaseous Hedrocarbons, a Survey of Thermophysical Properties of Compounds, No. 1 (63) (Institute of High Temperatures, Moscow, 1987).
B. A. Younglove and J. F. Ely,J. Phys. Chem. Ref. Data 16:577 (1987).
D. G. Friend, H. Ingham, and J. F. Ely,J. Phys. Chem. Ref. Data 20:275 (1991).
I. Hunter and E. B. Smith, personal communication (1989).
S. Hendl and E. Vogel,Fluid Phase Equil. 76:259 (1992).
E. Bich and E. Vogel,Int. J. Thermophys. 12:27 (1991).
J. Luettmer-Strathmann, S. Tang, and J. V. Sengers,J. Chem. Phys. 97:2705 (1992).
V. Vesovic and W. A. Wakeham, inCritical Fluid Technology, T. J. Bruno and J. F. Ely, eds. (CRC Press, Boca Raton, FL, 1991), Chap. 6.
R. Mostert, H. R. van den Berg, P. S. van der Gulik, and J. V. Sengers,J. Chem. Phys. 92:5454 (1990).
H. Vogel,Amt. Phys. 43:1235 (1914).
Y. Ishida,Phys. Rev. 21:550 (1923).
G. Martin,A Treatise on Chemical Engineering (Lockwood and Son, London, 1928).
T. Titani,Bull. Client. Soc. (Jap.) 5:98 (1930).
M. Trautz and K. G. Sorg,Ann. Phys. 10:81 (1931).
N. S. Rudenko and L. W. Schubnikow,Phys. Z. Sowjetunion 6:470 (1934) [data quoted by A. Van Itterbeek and O. van Paemel,Physica 8:133 (1941)].
H. Adzumi,Bull. Chem. Soc. (Jap.) 12:199 (1937).
S. F. Gerf and G. I. Galkov,Zhur. Tekh. Fiz. 10:725 (1940).
G. I. Galkov and S. F. Gerf,Zhur. Tekh. Fiz. 11:613 (1941).
S. F. Gerf and G. I. Galkov,Zhur. Tekh. Fiz. 11:801 (1941).
A. S. Smith and G. G. Brown,Ind. Eng. Chem. 35:705 (1943).
V. D. Majmudar and V. S. Oka,J. Univ. Bombay 17A:35 (1949).
P. M. Craven and J. D. Lambert,Proc. Rov. Soc. (London) 205A:439 (1951).
H. Senftleben,Z. Angew. Phys. 5:33 (1953).
N. V. Meshcheryakov and I. F. Golubev,Trudy GIAP 4:22 (1954).
J. D. Lambert, K. J. Cotton, M. W. Pailthorpe, A. M. Robinson, J. Scrivins, W. R. F. Vale, and R. M. Young,Proc. Roy. Soc. (London) 231A:280 (1955).
A. G. DeRocco and J. O. Halford,J. Chem. Phys. 28:1152 (1958).
J. D. Baron, J. G. Roof, and F. W. Wells,J. Chem. Eng. Data 4:283 (1959).
G. W. Swift, J. Lohrenz, and F. Kurata,AIChE J. 6:415 (1960).
K. E. Starling, B. E. Eakin, J. P. Dolan, and R. T. Ellington,Proc. 2nd Symp. Thermophys. Prop., P. F. Masi and D. H. Tsai, eds. (American Society of Mechanical Engineers, New York, 1962), p. 530.
B. E. Eakin, K. E. Starling, J. P. Dolan, and R. T. Ellington,J. Chem. Eng. Data 7:33 (1962); ADI Document No. 6856.
L. T. Carmichael and B. H. Sage,J. Chem. Eng. Data 8:94 (1963).
J. Kestin, S. T. Ro, and W. A. Wakeham,Trans. Faraday Soc. 67:2308 (1971).
H. J. Strumpf, A. F. Collings, and C. J. Pings,J. Chem. Phys. 60:3109 (1974).
M. Diaz Pena and J. A. R. Cheda,Anales Quint. (Espana) 71:34 (1975).
A. Cabello, F. Pedrosa, and M. Diaz Pena,Anales Quint. (Espana) 73:37 (1977).
J. Kestin, H. E. Khalifa, and W. A. Wakeham,J. Chem. Phys. 66:1132 (1977).
Y. Abe, J. Kestin, H. E. Khalifa, and W. A. Wakeham,Physica 93A:155 (1978).
H. Iwasaki and M. Takahashi,J. Chem. Phys. 74:1930 (1981).
D. E. Diller and J. M. Saber,Physica 108A:143 (1981).
D. E. Diller,Proc. 8th Symp. Thermophys. Prop., J. V. Sengers, ed. (American Society of Mechanical Engineers, New York, 1982), Vol. I, p. 219.
D. E. Diller and J. F. Ely,High Temp. High Press. 21:613 (1989).
R. D. Trengove and W. A. Wakeham,J. Phys. Chem. Ref. Data 16:175 (1987).
J. Millat, V. Vesovic, and W. A. Wakeham,Int. J. Thermophys. 12:265 (1991).
G. C. Maitland, M. Rigby, E. B. Smith, and W. A. Wakeham,Intermolecular Forces: Their Origin and Determination (Clarendon Press, Oxford, 1987).
S. Hendl, J. Millat, V. Vesovic, E. Vogel, and W. A. Wakeham,Int. J. Thermophys. 12:999 (1991).
CODATA Bulletin No. 63 (1986).
E. Bich, J. Millat, and E. Vogel,Wiss. Z. W.-Pieck-Univ. Rostock 36(N8):5 (1987).
A. Boushehri, J. Bzowski, J. Kestin, and E. A. Mason,J. Phys. Chem. Ref. Data 16:445 (1987);17:255 (1988).
J. V. Sengers,Int. J. Thermophys. 6:203 (1985).
S. L. Rivkin, A. Ya. Levin, L. B. Izrailevsky, and K. G. Kharitonov,Proc. 8th Int. Coral. Prop. Steam, P. Bury, H. Perdon, and B. Vodar, eds. (Editions Européennes Thermiques et Industries, Paris, 1975), p. 153.
R. S. Basu, J. V. Sengers, and J. T. R. Watson,Int. J. Thermophys. 1:33 (1980).
V. N. Zozulya and Y. P. Blagoi,Soc. Phys. JETP 39:99 (1974).
R. F. Berg and M. R. Moldover,Phys. Rev. A42:7183 (1990)
R. F. Berg and M. R. Moldover,J. Chem. Phys. 93:1926 (1990).
G. A. Olchowy and J. V. Sengers,Phys. Rev. Lett. 61:15 (1988).
R. Kraus, J. Luettmer-Strathmann, J. V. Sengers, and K. Stephan,Int. J. Thermophys. 14:951 (1993).
R. F. Berg and M. R. Moldover,J. Chem. Phys. 89:3694 (1988).
J. C. Nieuwoudt and J. V. Sengers,J. Chem. Phys. 92:5454 (1990).
H. Hao and R. A. Ferrell, in press.
D. G. Friend,J. Chem. Phys. 79:4533 (1983).
J. C. Rainwater,J. Chem. Phys. 81:495 (1984).
D. G. Friend and J. C. Rainwater,Chem. Phys. Lett. 107:590 (1984).
J. C. Rainwater and D. G. Friend,Phys. Rev. A36:4062 (1987).
M. Takahashi, C. Yokoyama, and S. Takahashi,Kagaku Kogaku Ronbunshu 11:155 (1985).
M. Takahashi, C. Yokoyama, and S. Takahashi,J. Chem. Eng. Data 32:98 (1987).
E. Vogel and S. Hendl,Fluid Phase Equil. 79:313 (1992).
H. J. M. Hanley, R. D. McCarty, and E. G. D. Cohen,Physica 60:322 (1972).
D. E. Diller and M. J. Ball,Int. J. Thermophys. 6:619 (1985).
K. M. de Reuck and B. Armstrong,Cryogenics 19:505 (1979).
M. J. Assael, E. Charitidou, J. H. Dymond, and M. Papadaki,Int. J. Thermophys. 13:237 (1992).
V. Vesovic, W. A. Wakeham, J. Luettmer-Strathmann, J. V. Sengers, J. Millat, E. Vogel, and M. J. Assael,Int. J. Thermophys. 15:33 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hendl, S., Millat, J., Vogel, E. et al. The transport properties of ethane. I. Viscosity. Int J Thermophys 15, 1–31 (1994). https://doi.org/10.1007/BF01439245
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01439245