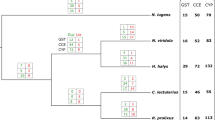
Abstract
Over 30 carboxylester hydrolases have been identified inD. melanogaster. Most are classified as acetyl, carboxyl or cholinesterases. Sequence similarities among most of the carboxyl and all the cholinesterases so far characterised fromD. melanogaster and other eukaryotes justify recognition of a carboxyl/cholinesterase multigene family. This family shows minimal sequence similarities with other esterases but crystallographic data for a few non-drosophilid enzymes show that the family shares a distinctive overall structure with some other carboxyl and aryl esterases, so they are all put in one superfamily of /β hydrolases. Fifteen esterase genes have been mapped inD. melanogaster and twelve are clustered at two chromosomal sites. The constitution of each cluster varies acrossDrosophila species but two carboxyl esterases in one cluster are sufficiently conserved that their homologues can be identified among enzymes conferring insecticide resistance in other Diptera. Sequence differences between two other esterases, the EST6 carboxyl esterase and acetylcholinesterase, have been interpreted against the consensus super-secondary structure for the carboxyl/cholinesterase multigene family; their sequence differences are widely dispersed across the structure and include substantial divergence in substrate binding sites and the active site gorge. This also applies when EST6 is compared across species where differences in its expression indicate a difference in function. However, comparisons within and among species where EST6 expression is conserved show that many aspects of the predicted super-secondary structure are tightly conserved. Two notable exceptions are a pair of polymorphisms in the substrate binding site of the enzyme inD. melanogaster. These polymorphisms are associated with differences in substrate interactionsvitro} and demographic data indicate that the alternative forms are not selectively equivalentin vivo.
Similar content being viewed by others
References
Abdel-Aal, Y. A. I., T. N. Hanzlik, B. D. Hammock, L. G. Harshman & G. Prestwich, 1988. Juvenile hormone esterases in two heliothines: kinetics, biochemical and immunogenic characterisation. Comp. Biochem. Physiol. 90B: 117–124.
Albuquerque, C. M. R. & M. Napp, 1981. Genetic variability at the esterase 6 locus in natural populations ofDrosophila simulans in relation to environmental heterogeneity. Genetics 98: 399–407.
Anderson, P. R. & J. G. Oakeshott, 1984. Parallel geographic patterns of allozyme variation in two siblingDrosophila species. Nature 308: 729–731.
Arnason, E. & G. K. Chambers, 1987. Macromolecular interaction and the electrophoretic mobility of esterase 5 fromDrosophila pseudoobscura. Biochem. Genet 25: 287–301.
Arpagaus, M., D. Fournier & J. P. Toutant, 1988. Analysis of acetyl cholinesterase molecular forms during the development ofDrosophila melanogaster. Evidence for the existence of an amphiphilic monomer. Insect Biochem. 18: 539–549.
Baker, W. K., 1980. Evolution of the alpha-esterase duplication within the montana subphylad of the virilis species group ofDrosophila. Genetics 94: 733–748.
Beckman, L. & F. M. Johnson, 1974. Esterase variations inDrosophila melanogaster. Hereditas 51: 212–220.
Berger, E. & R. Canter, 1973. The esterases ofDrosophila. I. The anodal esterases and their possible role in eclosion. Devel. Biol. 33: 48–55.
Brady, J. P. & R. C. Richmond, 1990. Molecular analysis of evolutionary changes in the expression ofDrosophila esterases. Proc. Natl. Acad. Sci. USA 87: 8217–8221.
Brady, J. P. & R. C. Richmond, 1992. An evolutionary model for the duplication and divergence of esterase genes inDrosophila. J. Mol. Evol. 34: 506–521.
Brady, J. P., R. C. Richmond & J. G. Oakeshott, 1990. Cloning of the esterase 5 locus fromDrosophila pseudoobscura and comparison with its homologue inDrosophila melanogaster. Mol. Biol. Evol. 7: 525–546.
Campbell, P. M., M. J. Healy & J. G. Oakeshott, 1992. Characterisation of juvenile hormone esterase inDrosophila melanogaster. Insect Biochem. Molec. Biol. 22: 665–677.
Cavener, D., D. C. Otteson & T. C. Kaufman, 1986. A rehabilitation of the genetic map of the 84B-D region ofDrosophila melanogaster. Genetics 114: 111–123.
Choi, K. D., G. H. Jeohn, J. S. Rhee & O. J. Yoo, 1990, Cloning and nucleotide sequence of an esterase gene fromPseudomonas fluorescens and expression of the gene inEschericia coli. Agric. Biol. Chem. 54: 2039–2045.
Chothia, C. & A. M. Lesk, 1986. The relationship between the divergence of sequence and structure in proteins. EMBO J. 5: 823–826.
Coates, P. M., M. A. Mestriner & D. A. Hopkinson, 1975. A preliminary genetic interpretation of the esterase isozymes of human tissues. Ann. Hum. Genet. 39: 1–20.
Cochrane, B. J. & R. C. Richmond, 1979. Studies of esterase-6 inDrosophila melanogaster. II. The genetics and frequency distributions of naturally occuring variants studied by electrophonesis and heat stability criteria. Genetics 93: 461–478.
Collet, C., K. M. Nielsen, R. J. Russell, M. Karl, J. G. Oakeshott & R. C. Richmond, 1990. Molecular analysis of duplicated esterase genes inDrosophila melanogaster. Molec. Biol. Evol. 7: 9–28.
Cooke, P. H. & J. G. Oakeshott, 1989. Amino acid polymorphisms for Esterase 6 inDrosophila melanogaster. Proc. Natl. Acad. Sci. USA 86: 1426–1430.
Cooke, P. H., R. C. Richmond & J. G. Oakeshott, 1987. High resolution electrophoretic variation at the Esterase-6 locus in a natural population ofDrosophila melanogaster. Heredity 59: 259–264.
Cygler, M., J. D. Schrag & F. Ergan, 1992. Advances in structural understanding of lipases. Biotech. Genet. Eng. Rev. 10: 143–184.
Cygler, M., J. D. Schrag, J. C. Sussman, M. Harel, I. Silman, M. K. Gentry & B. P. Doctor, 1993. Relationship between sequence conservation and three dimensional structure in a large family of esterases, lipases and related proteins. Protein Sci. 2: 366–382.
Danford, N. D. & J. A. Beardmore, 1979. Biochemical properties of esterase 6 inDrosophila melanogaster. Biochem. Genet. 17: 1–22.
Dayhoff, M. O., R. M. Schwartz & B. C. Orcutt, 1978. A model of evolutionary change in proteins, pp. 345–352 in Atlas of Protein Sequence and Structure, edited by M. O. Dayhoff. The National Biomedical Research Foundation, Silver Spring, Maryland.
De la Escalera, S., E.-O. Bockamp, F. Moya, M. Piovant & F. Jiménez, 1990. Characterisation and gene cloning of neurotactin, aDrosophila transmembrane protein related to cholinesterases. EMBO J. 9: 3593–3601.
Devonshire, A. L., 1977. The properties of a carboxylesterase from the peach-potato aphid,Myzus persicae (Sulz.), and its role in conferring insecticide resistance. Biochem. J. 167: 675–683.
Di Persio, L. P., R. N. Fontaine & D. Y. Hui, 1990. Identification of the active site serine in pancreatic cholesterol esterase by chemical modification and site-specific mutagenesis. J. Biol. Chem. 265: 16801–16806.
Di Persio, L. P., R. N. Fontaine & D. Y. Hui, 1991. Site-specific mutagenesis of an essential histidine residue in pancreatic cholesterol esterase. J. Biol. Chem. 266: 4033–4036.
Di Persio, L. P. & D. Y. Hui, 1993. Aspartic acid 320 is required for optimal activity of rat pancreatic cholesterol esterase. J. Biol. Chem. 268: 300–304.
East, P. D., 1982. Non-specific esterases ofDrosophila buzzatii, pp. 323–338, in Ecological Genetics and Evolution. The Cactus-Yeast-Drosophila Model System, edited by J. S. F. Barker and W. T. Starmer. Academic Press, New York.
East, P. D., 1984. Biochemical Genetics of Two Highly Polymorphic Esterases inDrosophila Buzzatii, 211 pp. PhD Thesis. University of New England, Armidale.
East, P. D., A. Graham & G. Whitington, 1990. Molecular isolation and preliminary characterisation of a duplicated esterase locus inDrosophila buzzatii, pp. 389–406 in Ecological and Evolutionary Genetics ofDrosophila, edited by J. S. F. Barker, W. T. Starmer and R. J. MacIntyre. Plenum Press, New York.
Ecobichon, D. J. & W. Kalow, 1965. Properties and classification of the soluble esterases of human skeletal and smooth muscle. Canad. J. Biochem. 43: 73–79.
Enikolopov, G. N., O. A. Malevanchuk, N. I. Peunova, P. V. Sergeev, & G. P. Georgiev, 1989. Est locus inDrosophila virilis contains two related genes. Doklady Akad. Nauk. SSSR 306: 1247–1249.
Farmer, J. L. & M. W. Carter, 1989. Properties of partially purified allozymes of esterase 5 ofDrosophila pseudoobscura. Comp. Biochem. Physiol. 93B: 451–458.
Farrell, D. H., P. Mikesell, L. A. Actis & J. H. Crosa, 1990. A regulatory gene, angR, of the iron uptake system ofVibrio anguillarum: similarity with phage P22 cro and regulation by iron. Gene 86: 45–51.
Felsenstein, J., 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791.
Felsenstein, J., 1991. Phylip. University of Washington, Seattle, Washington.
Field, L. M., M. S. Williamson, G. D. Moores & A. L. Devonshire, 1993. Cloning and analysis of the esterase genes conferring insecticide resistance in the peach-potato aphidMyzus persicae (Sulzer). Biochem. J. (in press).
Fournier, D., J.-B. Bergé, M.-L. C. de Almeida & C. Bordier, 1988. Acetylcholinesterases fromMusca domestica andDrosophila melanogaster brain are linked to membranes by a glycophospholipid anchor sensitive to an endogenous phospholipase. J. Neurochem. 50: 1158–1163.
Franklin, I. R., 1981. An analysis of temporal variation at isozyme loci inDrosophila melanogaster, pp. 217–236 in Genetic Studies ofDrosophila Populations, edited by J.B. Gibson and J. G. Oakeshott. The Australian National University Press, Canberra.
Game, A. Y. & J. G. Oakeshott, 1989. Variation in the amount and activity of Esterase 6 in a natural population ofDrosophila melanogaster. Heredity 62: 27–34.
Game, A. Y. & J. G. Oakeshott, 1990. Associations between restriction site polymorphism and enzyme activity variation for esterase 6 inDrosophila melanogaster. Genetics 126: 1021–1032.
Gan, K. N., A. Smolen, H. W. Eckerson & B. N. la Du, 1991. Purification of human serum paraoxonase/arylesterase: evidence for one esterase catalysing both activities. Drug Metabolism Disposition 19: 100–106.
Gibney, G., S. Camp, M. Dionne, K. Macphee-Quigley & P. Taylor, 1990. Mutagenesis of essential functional residues in acetylcholinesterase. Proc. Natl. Acad. Sci. USA 87: 7546–7550.
Healy, M. J., M. M. Dumancic & J.G. Oakeshott, 1991. Biochemical and physiological studies of soluble esterases fromDrosophila melanogaster. Biochem. Genet. 29: 365–388.
Heymann, E., 1980. Carboxylesterases and amidases, pp. 291–323 in Enzymatic Basis of Detoxication. Vol II, edited by W.B. Jakoby. Academic Press, New York.
Holmes, R. S. & C. J. Masters, 1967. The developmental multiplicity and isoenzyme status of cavian esterases. Biochim. Biophys. Acta 132: 379–399.
Hjoring, N. & O. Svensmark, 1988. Molecular and catalytic properties of a butyrylesterase from human red cells and brain. Arch. Biochem. Biophys. 260: 351–358.
Hui, D. Y., K. Hayakawa & J. Oizumi, 1993. Lipoamidase activity in normal and mutagenized pancreatic cholesterol esterase (bile salt stimulated lipase). Biochem J. 291: 65–69.
Jiang, C., J. B. Gibson & H. Chen, 1989. Genetic differentiation in populations ofDrosophila melanogaster from the People's Republic of China: comparison with patterns on other continents. Heredity 62: 193–198.
Johnson, F. M., C. G. Kanapi, R. H. Richardson, M. R. Wheeler & W. S. Stone, 1966. An operational classification ofDrosophila esterases for species comparison. Univ. Texas Publ. 6615: 517–532.
Johnson, F. M., R. H. Richardson & R. H. Kambysellis, 1968. Isozyme variability in species of the genusDrosophila. III. Qualitative comparison of the esterases ofD. aldrichi andD. mulleri. Biochem. Genet. 1: 239–247.
Kambysellis, M. P., F. M. Johnson & R. H. Richardson, 1968. Isozyme variability in species of the genusDrosophila. IV. Distribution of the esterases in the body tissues ofD. aldrichi andD. mulleri adults. Biochem. Genet. 1: 249–265.
Kao, L. R., N. Motoyama & W. C. Dauterman, 1985. The purification and characterisation of esterases from insecticide-resistant and susceptible house flies. Pestic. Biochem. Physiol. 23: 228–239.
Karotam, J. & J. G. Oakeshott, 1993. Regulatory aspects of esterase 6 activity variation in siblingDrosophila species. Heredity 71: 41–50.
Karotam, J., A. C. Delves & J. G. Oakeshott, 1993. Conservation and change in structural and 5′ flanking sequences of esterase 6 in siblingDrosophila species. Genetica 88: 11–28.
Karotam, J., T. M. Boyce & J. G. Oakeshott, 1994. Nucleotide variation at the hypervariable esterase 6 isozyme locus ofDrosophila simulans. Mol. Biol. Evol. (in press).
Korochkin, L. I., M. Z. Ludwig, E. V. Poliakova & M. R. Philinova, 1987. Some molecular genetic aspects of cellular differentiation inDrosophila. Sov. Sci. Rev. F. Physiol. Gen. Biol. 1: 411–466.
Korochkin, L., M. Ludwig, N. Tamarina, I. Uspensky, G. Yenikolopov, R. Khechumijan, M. Kopantseva, M. Evgeniev, B. Kuzin, T. Bakayeva, L. Mudjoian, O. Malevantschuk, V. Tsatyian, A. Ivanov & S. Lukianov, 1990. Molecular genetic mechanisms of tissue specific esterase isozymes and protein expression inDrosophila, pp. 399–440 in Isozymes: Structure, Function and Use in Biology and Medicine, edited by C. Markert and J. Scandalios. Wiley-Liss, New York.
Korochkin, L. I., N. M. Matveeva & A. Yu. Kerkis, 1973. Subcellular localisation of esterases inD. virilis andD. texana. Dros. Inform. Serv. 50: 130–131.
Labate, J., A. Bortoli, A. Y. Game, P. H. Cooke & J. G. Oakeshott, 1989. Further observations on the number and distribution of esterase 6 alleles in populations ofDrosophila melanogaster. Heredity 63: 203–208.
Lai-Fook, J. & P. H. Smith, 1991. Genetics of the hemolymph esterases ofLucilia cuprina (Diptera: Calliphoridae). Biochem. Genet. 30: 123–130.
Loukas, M., 1981. A new esterase locus inD. melanogaster. Dros. Inform. Serv. 56: 85.
Ludwig, M. Z., N. A. Tamarina & R. C. Richmond, 1993. Localisation of sequences controlling the spatial, temporal and sex-specific expression of the esterase 6 locus inDrosophila melanogaster adults. Proc. Natl. Acad. Sci. USA 90: 6233–6237.
Luthi, E., D. R. Love, J. McAnulty, C. Wallace, P. A. Caughey, D. Saul & P. C. Bergquist, 1990. Cloning, sequence analysis and expression of genes encoding xylan-degrading enzymes from the thermophileCaldocellum saccharolyticum. Appl. Env. Microbiol. 56: 1017–1024.
Mane, S. D., C. S. Tepper & R. C. Richmond, 1983. Studies of esterase 6 inDrosophila melanogaster. XIII. Purification and characterisation of the two major isozymes. Biochem. Genet. 21: 1019–1040.
Markovic, O. & H. Jornvall, 1986. Pectinesterase: the primary structure of the tomato enzyme. Eur. J. Biochem. 158: 455–462.
Mercken, L., M.-J. Simmons, S. Swillens, M. Massaer & G. Vassart, 1985. Primary structure of bovine thyroglobulin deduced from the sequence of its 8431-base complementary DNA. Nature 316: 647–651.
Miekle, D. B. & R. C. Richmond, 1990. Localisation and longevity of seminal fluid esterase 6 in mated femaleDrosophila melanogaster. J. Insect Physiol. 36: 93–101.
Morton, R. A. & R. S. Singh, 1985. Biochemical properties, homology, and genetic variation ofDrosophila non-specific esterases. Biochem. Genet. 23: 959–973.
Mouchés, C., M. Magnin, J.-P. Bergé, M. de Silvestri, V. Beyssat, N. Pasteur & G. P. Georghiou, 1987. Overproduction of detoxifying esterases in organophosphate-resistantCulex mosquitoes and their presence in other insects. Proc. Natl. Acad. Sci. USA 84: 2113–2116.
Mulley, J. C., J. W. James & J. S. F. Barker, 1979. Allozyme genotype-environment relationships in natural populations ofDrosophila buzzatii. Biochem. Genet. 17: 105–126.
Myers, M. A., M. J. Healy & J. G. Oakeshott, 1993. Effects of the residue adjacent to the reactive serine on the substrate interactions of Drosophila esterase 6. Biochem. Genet. 31: 259–278.
Myers, M. A., M. J. Healy & J. G. Oakeshott, 1994. Effects of four N-linked glycans on thein vivo function of esterase 6 inD. melanogaster (submitted for publication).
Narise, S., 1973. Esterase isozymes ofDrosophila virilis: purification and properties of α- and β-esterase isozymes. Japan. J. Genet. 48: 119–132.
Narise, S. & J. L. Hubby, 1966. Purification of esterase 5 fromDrosophila pseudoobscura. Biochem. Biophys. Acta. 122: 281–288.
Nei, M., 1987. Molecular Evolutionary Genetics. Columbia University Press, New York.
Oakeshott, J. G., G. K. Chambers, J. B. Gibson & D. A. Willcocks, 1981. Latitudinal relationships of esterase-6 and phosphoglucomutase gene frequencies inDrosophila melanogaster. Heredity 47: 385–396.
Oakeshott, J. G., C. Collet, R. Phillis, K. M. Nielsen, R. J. Russell, G. K. Chambers, V. Ross & R. C. Richmond, 1987. Molecular cloning and characterisation of Esterase 6, a serine hydrolase fromDrosophila. Proc. Natl. Acad. Sci. USA 84: 3359–3363.
Oakeshott, J. G., P. H. Cooke, R. C. Richmond, A. Bortoli, A. Y. Game & J. Labate, 1989. Molecular population genetics of structural variants of esterase 6 inDrosophila melanogaster. Genome 31: 788–797.
Oakeshott, J. G., M. J. Healy & A. Y. Game, 1990. Regulatory evolution of β-carboxyl esterases inDrosophila, pp. 359–387 in Ecological and Evolutionary Genetics of Drosophila, edited by J. S. F. Barker, W. T. Starmer and R. J. MacIntyre. Plenum, New York.
Oakeshott, J. G., M. Saad, A. Y. Game & M. J. Healy, 1994. Causes and consequences of esterase 6 enzyme activity variation in pre-adultDrosophila melanogaster (submitted for publication).
Oakeshott, J. G., S. R. Wilson & W. R. Knibb, 1988. Selection affecting enzyme polymorphisms in confined populations ofDrosophila living in a natural environment. Proc. Natl. Acad. Sci. USA 85: 293–297.
O'Brien, S. J., 1990. Genetic Maps: Locus maps of Complex Genomes. Cold Spring Harbor Laboratory Press, New York.
Olson, P. F., L. I. Fessler, R. E. Nelson, R. E. Sterne, A. G. Campbell & J. H. Fessler, 1990. Glutactin, a novelDrosophila basement membrane-related glycoprotein with a sequence similarity to serine esterases. EMBO J. 9: 1219–1227.
Okada, Y. & K. Wakabayashi, 1988. Purification and characterisation of esterases D-1 and D-2 from human erythrocytes. Arch. Biochem. Biophys. 263: 130–136.
Ollis, D. L., E. Cheah, M. Cygler, B. Dijkstra, F. Frolow, S. M. Franken, M. Harel, S. J. Remington, I. Silman, J. Schrag, J. L. Sussman, K. H. G. Verschueren & A. Goldman, 1992. The α/β hydrolase fold. Protein Eng. 5: 197–211.
Oppenoorth, F. J. & K. van Asperen, 1960 Allelic genes in the housefly producing modified enzymes that cause organophosphate resistance. Science 132: 298–299.
Ounissi, H. & P. Courvalin, 1985. Nucleotide sequence of the gene ereA encoding the erythromycin esterase inEschericia coli. Gene 35: 271–278.
Parker, A. G., R. J. Russell, A. C. Delves & J. G. Oakeshott, 1991. Biochemistry and physiology of esterases in organophosphate-susceptible and-resistant strains of the Australian Sheep Blowfly,Lucilia cuprina. Pestic. Biochem. Physiol. 41: 305–318.
Pen, J., H. A. H. Rongen & J. J. Beintema, 1984. Purification and properties of esterase 4 fromDrosophila mojavensis. Biochim. Biophys. Acta. 789: 203–209.
Pen, J., J. van Beeumen & J. J. Beintema, 1986. Structural comparison of two esterases fromDrosophila mojavensis isolated by immunoaffinity chromatography. Biochem. J. 278: 691–699.
Piovant, M., I. Darboux, Y. Barthalay & R. Hipeau-Jacquotte, 1993.Drosophila neurotactin, an heterophilic cell recognition molecule related to cholinesterases: structural and functional characterisation of the extracellular domain, p. 74. Proc. XXth North Amer. Dros. Conf. (abstract).
Procunier, W. S., J. J. Smith & R. C. Richmond, 1991. Physical mapping of the Esterase-6 locus ofDrosophila melanogaster. Genetica 84: 203–208.
Raftos, D. A. & P. B. Hughes, 1986. Genetic basis of a specific resistance to malathion in the Australian sheep blowfly,Lucilia cuprina (Diptera: Calliphoridae). J. Econ. Entomol. 79: 553–557.
Ray, J., J. Knapp, D. Grierson, C. Bird & W. Schuch, 1988. Identification and sequence determination of a cDNA clone for tomato pectin esterase. Eur. J. Biochem. 174: 119–124.
Raymer, G., J. M. A. Willard & J. L. Schottel, 1990. Cloning, sequencing and regulation of expression of an extracellular esterase gene from the plant pathogenStreptomyces scabies. J. Bacteriol 172: 7020–7026.
Richmond, R. C., K. M. Nielsen, J. P. Brady & E. M. Snella, 1990. Physiology, biochemistry and molecular biology of theEst6 locus inDrosophila melanogaster, pp. 273–292 in Ecological and Evolutionary Genetics ofDrosophila, edited by J.S.F. Barker, W.T. Starmer and R.J. MacIntyre: Plenum, New York.
Rippol, D. R., C. H. Faerman, P. H. Axelsen, I. Silman & J. L. Sussman, 1993. An electrostatic mechanism for substrate guidance down the aromatic gorge of acetylcholinesterase. Proc. Natl. Acad. Sci. USA 90: 5128–5132.
Ruddle, F. H. & L. Harrington, 1967. Tissue specific isozymes of the mouse (Mus Musculus). J. Exp. Zool. 166: 51–64.
Russell, R. J., M. M. Dumancic, G.G. Foster, G.L. Weller, M.J. Healy & J.G. Oakeshott, 1990. Insecticide resistance as a model system for studying molecular evolution, pp. 293–314 in Ecological and Evolutionary Genetics ofDrosophila, edited by J.S.F. Barker, W.T. Starmer and R.J. MacIntyre. Plenum, New York.
Saad, M., A. Y. Game, M. J. Healy & J. G. Oakeshott, 1994. Associations of esterase 6 allozyme and activity variation with reproductive fitness inDrosophila melanogaster (submitted for publication).
Saitou, N. & M. Nei, 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425.
Sander, D. & R. Schneider, 1991. Database of homology-derived protein structures. Proteins 9: 56–68.
Sasaki, F., 1974. Esterase isozymes ofDrosophila virilis: developmental aspects of the α- and β-esterases and their distribution in the body parts. Japan. J. Genet. 49: 223–232.
Sasaki, F., 1975. Immunological comparison of the α- and β-esterases inDrosophila species. Japan. J. Genet. 50: 217–233.
Sasaki, M. & S. Narise, 1978. Molecular weight of esterase isozymes ofDrosophila species. Dros. Inform. Serv. 53: 123–124.
Schafer, D. J., D. K. Fredline, W. R. Knibb, M. M. Green & J. S. F. Barker, 1993. Genetics and linkage mapping ofDrosophila buzzatii. Heredity (in press).
Schrag, J. D. & M. Cygler, 1993. The 1.8 Å refined structure of lipase fromGeotrichum candidum. J. Mol. Biol. 230: 575–591.
Sergeev, P. V., G. N. Yenikolopov, N. I. Peunova, B. A. Kuzin, R. A. Khechumian, L. I. Korochkin & G. P. Georgiev, 1993. Regulation of tissue specific expression of the esterase S gene inDrosophila virilis. Nucl. Acids Res 21: 3545–3551.
Smyth, K. A., V. K. Walker, J. G. Oakeshott & R. J. Russell, 1994. Biochemical and genetic characterisation of malathion resistance inLucilia cuprina (in preparation).
Spackman, M. E., J. G. Oakeshott, K.-A. Smyth, K. M. Medveczky & R. J. Russell, 1993. A cluster of esterase genes on chromosome 3R ofDrosophila melanogaster includes homologues of esterase genes conferring insecticide resistance in severalDiptera (submitted for publication).
Spierer, P., A. Spierer, w. Bender & D. S. Hogness, 1983. Molecular mapping of genetic and chromomeric units inDrosophila melanogaster. J. Mol. Biol. 168: 35–50.
Sreerama, L. & P. S. Veerabhadrappa, 1991. Purification and properties of carboxylesterases from the termiteOdentotermes horni. W. Insect Biochem. 21: 833–844.
Sussman, J. S., M. Harel, F. Frolor, C. Oefner, A. Goldman, L. Toker & I. Silman, 1991. Atomic structure of acetylcholinesterase fromTorpedo californica: a prototypic acetylcholine-binding protein. Science 253: 872–879.
Taylor, W. R., 1986. The classification of amino acid conservation. J. Theor. Biol. 119: 205–218.
Triantaphyllidis, C. D. & C. Christodoulou, 1973. Studies of a homologous gene-enzyme system, esterase C, inDrosophila melanogaster andDrosophila simulans. Biochem. Genet. 8: 383–390.
Tsuno, K., N. T. Aotsuka & S. Ohba, 1984. Further genetic variation at the esterase loci ofDrosophila virilis. Biochem. Genet. 22: 323–337.
Van der Meer, J. R., R. I. L. Eggen, A. J. B. Zehnder & W. M. de Vos, 1991. Sequence analysis of thePseudomonas sp. strain P51 tcb gene cluster, which encodes metabolism of chlorinated catechols: evidence for specialisation of catechol 1,2-dioxygenases for chlorinated substrates. J. Bacteriol. 173: 2425–2434.
Van Tilbeurgh, H., M.-P. Egloff, C. Martinez, N. Rugani, R. Verger & C. Cambillau, 1993. Interfacial activation of the lipase-procolipase complex by mixed micelles revealed by X-ray crystallography. Nature 362: 814–820.
Von Deimling, O. & A. Gaa, 1992. Esterase-29 (Es29): biochemical characterisation and control by two independent gene loci of a testosterone-dependent mouse serum esterase. Biochem. Genet. 30: 421–436.
Von Deimling, O. & B. Wassmer, 1991. Genetic characterisation of esterase 28 (Es28) of the house mouse. Biochem. Genet. 29: 5–9.
Vellom, D. C., Z. Radi'c, Y. Li, N. A. Pickering, S. Camp & P. Taylor, 1993. Amino acid residues controlling acetylcholinesterase and butyrylcholinesterase specificity. Biochemistry 32: 12–17.
White, M. M., S. D. Mane & R. C. Richmond, 1988. Studies of esterase 6 inDrosophila melanogaster. XVIII. Biochemical differences between the slow and fast allozymes. Mol. Biol. Evol. 5: 41–62.
Ziegler, R., S. Whyard, A. E. R. Downe, G. R. Wyatt & V. K. Walker, 1987. General esterase, malathion carboxylesterase, and malathion resistance inCulex tarsalis. Pestic. Biochem. Physiol. 28: 279–285.
Zouros, E. & W. van Delden, 1982. Substrate preference polymorphism at an esterase locus ofDrosophila mojavensis. Genetics 100: 307–314.
Zouros, E., W. van Delden, R. Odense & H. van Dijk, 1982. An esterase duplication inDrosophila; differences in expression of duplicate loci within and among related species. Biochem. Genet. 20: 929–942.
Zschunke, F., A. Salmassi, H. Kreipe, F. Buck, M. R. Parwaresch & H. J. Radzun, 1991. cDNA cloning and characterisation of human monocyte/macrophage serine esterase-1. Blood 78: 506–512.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oakeshott, J.G., van Papenrecht, E.A., Boyce, T.M. et al. Evolutionary genetics ofDrosophila esterases. Genetica 90, 239–268 (1993). https://doi.org/10.1007/BF01435043
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01435043