Abstract
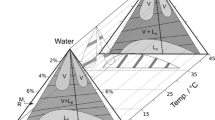
Partial phase diagrams showing the domains of existence of a transparent, viscous, lamellar-structured (D)-phase that transforms reversibly into fluid single phase solutions at high temperature are presented for the system: cetyltrimethylammonium bromide (CTAB), two low molecular weight alcohols, and water with and without additives. At constant temperature and with a fixed amount of surfactant, the size and location of this phase in the phase diagram depends upon three composition variables: i) the ratio of concentrations of medium chain alcohol to long chain alcohol (R), ii) the ratio of concentrations of medium chain alcohol to surfactant (R′), and iii) the concentrations of small amounts (up to 10 % by weight) of additives such as ethylene glycol, propylene glycol, and dimethylformamide, as well as NaBr. Small-angle x-ray scattering measurements of these mixtures reveal a lamellar structure. The observed lamellar repeat distances range from 60 A to 290 Å and depend upon the ratiosR andR′ and the concentration of the additives. The mechanical and structural properties of theseD-phases can be tuned by adjustingR andR′. TheD-phase-to-isotropic transition temperature can be varied from near room temperature to above 80 °C by adjustingR andR′.
Similar content being viewed by others
References
Degiorgio V, Corti M (eds) (1985) Physics of Amphiphiles. North Holland Publishing, Amsterdam
Israelachvili JN (1985) Intermolecular and Surface Forces. Academic Press, Orlando
Mittal KL, Botherel P (eds) (1984) Surfactant Solutions. Plenum Press, New York, vols 4–6
Israelachvili JN, Mitchell DJ, Ninham BW (1976) J Chem Soc Far Trans II 72:1525
Tanford C (1980) The Hydrophobic Effect: Formation of Micelles and Biological Membranes. John Wiley, New York
Mitchell DJ, Ninham BW (1981) J Chem Soc Far Trans II 77:601
Gelbart WM, Ben-Shaul A, McMullen WE, Masters A (1984) J Phys Chem 88:861
Bartouno R, Meuti M, Chidichimo G, Ranier GA (1985) In: Degiorgio V, Corti M (eds) Physics of Amphiphiles. North Holland Publishing, Amsterdam, p 524
Fontell K (1974) In: Gray GW, Winsor P (eds) Liquid Crystals and Plastic Crystals. Ellis Horwood London, vol 2, Ch 4
Porte G, Gomati R, El Haitamy O, Appell J, Marignan J (1986) J Phys Chem 90:5746
Ekwall P (1975) Adv Liq Cryst 1:1
Winsor P (1974) In: Gray GW, Winsor P (eds) Liquid Crystals and Plastic Crystals. Halsted Press, vol 1, p 193
Hirsch E, Wittmann JC, Candau F (1982) J Disp Sci Technol 3:351
Friberg S, Liang P (1986) Colloid Polym Sci 264:449
Ekwall P, Danielsson I, Mandell L (1960) Kolloid Z 169:113
Fontell K, Mandell L, Lehtinen H, Ekwall P (1968) Acta Polytech Scand Chem Incl Met Sci 74:III
Mandell L, Ekwall P (1968) Acta Polytech Chem Incl Met Sci 74:II
Ekwall P, Mandell L, Fontell K (1969) J Colloid Interface Sci 31:508
Ekwall P, Mandell L, Fontell K (1969) Mol Cryst Liq Cryst 8:157
Ekwall P, Mandell L, Fontell K (1970) J Colloid Interface Sci 33:215
Fontell K (1973) J Colloid Interface Sci 43:156
Parker AJ (1962) Quart Rev 16:163
Ionescu LG, Romanesco LS, Nome F (1984) In: Mittal KL, Lindman B (eds) Surfactants in Solution. Plenum Press, New York, vol 2, p 789
Parker AJ (1969) Chem Rev 69:1
Bougard J, Jadot R (1975) J Chem Thermodyn 7:1185
Berry BW, Saunders GM (1970) J Colloid Interface Sci 34:300
Patel HK, Rowe RC, McMahon J, Stewart RF (1985) Int J Pharm 25:13
de Vringer T, Joosten JGH, Junginger H (1984) Colloid Polym Sci 262:56
de Vringer T, Joosten JGH, Junginger H (1987) Colloid Polym Sci 265:167
Lipowsky R, Leibler S (1986) Phys Rev Letts 56:2541
Lipowsky R, Leibler S (1987) Phys Rev 35B:7004
Helfrich W (1978) Z Naturforsch 33a:705
Ross PA (1926) Phys Rev 28:425; (1928) J Opt Soc Amer 16:375 & 433
Vonk CC (1982) In: Glatter O, Kratky O (eds) Small Angle X-ray Scattering. Academic Press, New York, p 433
Burge RE, Draper JC (1967) Act Cryst 22:6
Lang J, Zana R (1986) J Phys Chem 90:5258
Ericksson J, Henriksson U, Klason T, Odberg L (1982) In: Mittal KL, Fendler E (eds) Solution Behavior of Surfactants. Plenum Press, New York, vol 2, p 907
de Gennes PG, Taupin C (1982) J Phys Chem 86:2294
Di Meglio JM, Dvolaitzky M, Taupin C (1985) J Phys Chem 89:871
Douzou P, Hui Bow Hoa G, Maurel P, Travers F (1976) In: Fasman GD (ed) CRC Handbook of Biochemistry and Molecular Biology. 3rd edition, CRC Press, Ohio, vol 1, pp 520–528
El-Nokaly MA, Ford LD, Friberg SE, Larsen DW (1981) J Colloid Interface Sci 84:228
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murthy, K.A., Kaler, E.W. Lamellar phases in mixtures of low molecular weight alkanols and cetyltrimethylammonium bromide (CTAB). Colloid & Polymer Sci 267, 330–335 (1989). https://doi.org/10.1007/BF01413626
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01413626