Summary
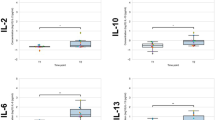
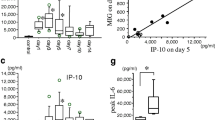
To elucidate the role of cytokines in brain repair processes and in local inflammation after neurosurgical procedures, cerebrospinal fluid (CSF) samples from 8 patients with intra-axial tumours and 8 patients with extra-axial tumours were analysed for interleukin (IL)-1beta, IL-1 receptor antagonist (IL-1ra), IL-6, IL-8, IL-10, and tumour necrosis factor (TNF)-alpha at the beginning and after surgery. Levels of IL-6 and IL-8 increased dramatically in all patients just hours after surgery and fell during subsequent days. IL-1beta was found only in low amounts in the CSF of both patient groups. Other cytokines demonstrated different courses. In patients with intra-axial tumours IL-1ra peaked two to four hours after surgery with a subsequent decrease. In patients with extra-axial tumours there was a continuous low-level IL-1ra release into the CSF without a peak. TNF-alpha was not present in detectable levels in the CSF after surgery for extra-axial tumours but was found to peak two to four hours after surgery for intra-axial tumours. IL-10 was detected in the CSF of both patient groups, but a higher peak was seen after surgery for extra-axial tumours. These results suggest different requirements for the cytokine response and an involvement of different cell types in cytokine release. However, the analysis of the CSF from both patient groups showed no differences in cell counts and populations, with a mild pleocytosis being present in both patient groups after surgery. Therefore, we conclude that after surgery for extra-axial tumours cytokines were predominately produced by non-immune cells stimulated through hypoxia or mechanical irritation. After surgery for intra-axial tumours with a significant brain injury immune cells — activated by necrotic material —seem to be involved in the process of cytokine synthesis. In these cases an additional IL-1ra and TNF-alpha peak was found and these cytokines may be markers for cerebral injury.
Similar content being viewed by others
References
Aloisi F, Carè A, Borsellino G, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C (1995) Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol 149 (1992): 2358–2366
Asadullah K, Woiciechowsky C, Döcke WD, Liebenthal C, Wauer H, Volk HD, Kox W, Vogel S, von Baehr R (1995) Immunodepression following neurosurgical procedures. Crit Care Med 23: 1976–1983
Asadullah K, Woiciechowsky C, Döcke WD, Egerer K, Kox W, Vogel S, Sterry W, Volk HD (1996) Very low monocytic HLA-DR expression indicates high risk of infection — immunomonitoring for patients after neurosurgery and patients during high dose steroid therapy. Eur J Emerg Med 2: 184–190
Balasingam V, Yong VW (1996) Attenuation of astroglial reactivity by interleukin-10. J Neurosci 16: 2945–2955
Cassatella MA, Meda L, Gasperini S, Calzetti F, Bonora S (1994) Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med 179: 1695–1699
Clark WC, Bressler J (1988) Transforming growth factor-beta-like activity in tumors of the central nervous system. J Neurosurg 68: 920–924
Daftarian PM, Kumar A, Kryworuchko M, Diaz Mitoma F (1996) IL-10 production is enhanced in human T cells by IL-12 and IL-6 and in monocytes by tumor necrosis factor-alpha. J Immunol 157: 12–20
Damme Jv (1994) Interleukin-8 and related chemotactic cytokines. In: Thomson A (ed) The cytokine handbook. 2nd ed. London, pp 185–208
Elliott LH, Brooks WH, Roszman TL (1984) Cytokinetic basis for the impaired activation of lymphocytes from patients with primary intracranial tumors. J Immunol 132: 1208–1215
Fontana A, Hengartner H, de Tribolet N, Weber E (1984) Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol 132: 1837–1844
Giulian D, Lachman LB (1985) Interleukin-1 stimulation of astroglial proliferation after brain injury. Science 228: 497–499
Halstensen A, Ceska M, Brandtzaeg P, Redl H, Naess A, Waage A (1993) Interleukin-8 in serum and cerebrospinal fluid from patients with meningococcal disease. J Infect Dis 167: 471–475
Hoch RC, Rodriguez R, Manning T, Bishop M, Mead P, Shoemaker WC, Abraham E (1993) Effects of accidental trauma on cytokine and endotoxin production [see comments]. Crit Care Med 21: 839–845
Karakurum M, Shreeniwas R, Chen J, Pinsky D, Yan SD, Anderson M, Sunouchi K, Major J, Hamilton T, Kuwabara K,et al (1994) Hypoxic induction of interleukin-8 gene expression in human endothelium cells. J Clin Invest 93: 1564–1570
Kuppner MC, Hamou MF, Sawamura Y, Bodmer S, de Tribolet N (1989) Inhibition of lymphocyte function by glioblastoma-derived transforming growth factor beta 2. J Neurosurg 71: 211–217
Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW (1993) Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol 150: 2659–2667
Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ (1994) Tumor necrosis factor-alpha expression in ischemic neurons. Stroke 25: 1481–1488
Maeda Y, Matsumoto M, Hori O, Kuwabara K, Ogawa S, Yan SD, Ohtsuki T, Kinoshita T, Kamada T, Stern DM (1994) Hypoxia/reoxygenation-mediated induction of astrocyte interleukin 6: a paracrine mechanism potentially enhancing neuron survival. J Exp Med 180: 2297–2308
Maimone D, Annunziata P, Simone IL, Livrea P, Guazzi GC (1993) Interleukin-6 levels in the cerebrospinal fluid and serum of patients with Guillain-Barre syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J Neuroimmunol 47: 55–61
Maimone D, Cioni C, Rosa S, Macchia G, Aloisi F, Annunziata P (1993) Norepinephrine and vasoactive intestinal peptide induce IL-6 secretion by astrocytes: synergism with IL-1 beta and TNF alpha. J Neuroimmunol 47: 73–81
Mathiesen T, Andersson B, Loftenius A, von Holst H (1993) Increased interleukin-6 levels in cerebrospinal fluid following subarachnoid hemorrhage. J Neurosurg 78: 562–567
McClain CJ, Cohen D, Ott L, Dinarello CA, Young B (1987) Ventricular fluid interleukin-1 activity in patients with head injury. J Lab Clin Med 110: 48–54
McClain C, Cohen D, Phillips R, Ott L, Young B (1991) Increased plasma and ventricular fluid interleukin-6 levels in patients with head injury [see comments]. J Lab Clin Med 118: 225–231
Meisel C, Vogt K, Platzer C, Randow F, Liebenthal C, Volk HD (1996) Differential regulation of monocytic tumor necrosis factor-alpha and interleukin-10 expression. Eur J Immunol 26: 1580–1586
Morganti Kossmann MC, Kossmann T, Brandes ME, Mergenhagen SE, Wahl SM (1992) Autocrine and paracrine regulation of astrocyte function by transforming growth factor-beta. J Neuroimmunol 39: 163–173
Nitta T, Hishii M, Kiyoshi S, Okumura K (1994) Selective expression of interleukin-10 gene within glioblastoma multiforme. Brain Res 649: 122–128
O'Nuallain EM, Puri P, Reen DJ (1993) Early induction of IL-1 receptor antagonist (IL-1Ra) in infants and children undergoing surgery. Clin Exp Immunol 93: 218–222
O'Nuallain EM, Puri P, Mealy K, Reen DJ (1995) Induction of interleukin-1 receptor antagonist (IL-1ra) following surgery is associated with major trauma. Clin Immunol Immunopathol 76: 96–101
Platzer C, Meisel C, Vogt K, Platzer M, Volk HD (1995) Upregulation of monocytic IL-10 by tumor necrosis factor-alpha and cAMP elevating drugs. Int Immunol 7: 517–523
Ramilo O, Saez Llorens X, Mertsola J, Jafari H, Olsen KD, Hansen EJ, Yoshinaga M, Ohkawara S, Nariuchi H, McCracken GH (1990) Tumor necrosis factor alpha/cachectin and interleukin 1 beta initiate meningeal inflammation. J Exp Med 172: 497–507
Randow F, Syrbe U, Meisel C, Krausch D, Zuckermann H, Platzer C, Volk HD (1995) Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J Exp Med 181: 1887–1892
Relton JK, Rothwell NJ (1992) Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res Bull 29: 243–246
Renno T, Lin JY, Piccirillo C, Antel J, Owens T (1994) Cytokine production by cells in cerebrospinal fluid during experimental allergic encephalomyelitis in SJL/J mice. J Neuroimmunol 49: 1–7
Roszman TL, Brooks WH, Elliott LH (1987) Inhibition of lymphocyte responsiveness by a glial tumor cell-derived suppressive factor. J Neurosurg 67: 874–879
Sharief MK, Thompson EJ (1992) In vivo relationship of tumor necrosis factor-alpha to blood-brain barrier damage in patients with active multiple sclerosis. J Neuroimmunol 38: 27–33
Tada M, de Tribolet N (1993) Recent advances in immunobiology of brain tumors. J Neurooncol 17: 261–271
Tada M, Sawamura Y, Sakuma S, Suzuki K, Ohta H, Aida T, Abe H (1993) Cellular and cytokine responses of the human central nervous system to intracranial administration of tumor necrosis factor alpha for the treatment of malignant gliomas. Cancer Immunol Immunother 36: 251–259
Tada M, Diserens AC, Desbaillets I, Jaufeerally R, Hamou MF, de Tribolet N (1994) Production of interleukin-1 receptor antagonist by human glioblastoma cells in vitro and in vivo. J Neuroimmunol 50: 187–194
Tracey KJ (1996) Tumour necrosis factor-alpha. In: Thomson A (ed) The cytokine handbook. 2nd ed. London, pp 289–304
Van Meir EG (1995) Cytokines and tumors of the central nervous system. Glia 15: 264–288
Wanidworanum C, Strober W (1993) Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol 151: 6853–6861
Woiciechowsky C, Asadullah K, Schöning B, Nestler D, Vogel S, Döcke WD, Volk HD (1996) Neurosurgery induces only local signs of inflammation but systemic antiinflammation and immunodepression: role of neuroendocrine stress response. [Abstract] Immunobiology 196: 86
Yamauchi Takihara K, Ihara Y, Ogata A, Yoshizaki K, Azuma J, Kishimoto T (1995) Hypoxic stress induces cardiac myocyte-derived interleukin-6. Circulation 91: 1520–1524
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Woiciechowsky, C., Asadullah, K., Nestler, D. et al. Different release of cytokines into the cerebrospinal fluid following surgery for intra- and extra-axial brain tumours. Acta neurochir 139, 619–624 (1997). https://doi.org/10.1007/BF01411996
Issue Date:
DOI: https://doi.org/10.1007/BF01411996