Summary
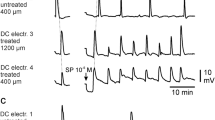
The feline infusion model of brain edema was used to evaluate the role of bradykinin in the etiology and pathophysiology of vasogenic brain edema. Bradykinin (3 or 90 ug in 600 μL saline) did not alter normocapnic regional cerebral blood flow (rCBF) nor induce specific changes in either the somatosensory (SEP) or motor (MEP) evoked potentials. The mean increases in ICP (from 4.5 to 16.1 mmHg) and peri-infusion white matter water content (from 69.4 to 79.8 ml/100 g tissue), mean decrease in lumped craniospinal compliance (from 0.040 to 0.014 ml/mmHg) and local histological changes were all similar to those after 600 μL saline infusion. The interstitial bradykinin infusion caused focal blood-brain-barrier (BBB) opening to Evans Blue dye and was chemotaxic for granulocytes. After the infusion there was a global loss of rCBF CO2 reactivity but there was no ischemia at normocapnia. These results show that bradykinin in brain edema fluid, at concentrations greater than those found in neuropathological conditions, can open the BBB of normal cerebral parenchymal capillaries and cause vascular dysregulation. In neuropathological conditions bradykinin may therefore potentiate formation of vasogenic brain edema but does not contribute to perilesional brain dysfunction.
Similar content being viewed by others
References
Baethmann A, Oettinger W, Rothenfusser Oet al (1980) Brain edema factors: Current state with particular reference to plasma constituents and glutamate. Adv Neurology 28: 171–195
Bodsch W, Rommel T, Ophoff BGet al (1987) Factors responsible for the retention of fluid in human tumour edema and the effect of dexamethasone. J Neurosurg 67: 250–257
Clark MA, Bomalaski JS, Conway TMet al (1986) Differential effects of apsirin and dexamethasone on phospholipase A2 and C activities and arachidonic acid release from endothelial cells in response to bradykinin and leukotriene D4. Prostaglandins 32: 703–708
Czernicki Z (1979) Treatment of experimental brain edema following sudden decompression, surgical wound and cold lesion with vasopressor drugs and proteinase inhibitor trasylol. Acta Neurochir (Wien) 50: 311–326
Dacey RG, Bassett JE, Takayasu M (1988) Vasomotor responses of rat intracerebral arterioles to vasoactive intestinal peptide, Substance P, Neuropeptide Y and bradykinin. J Cerebral Blood Flow Metab 8: 254–261
Deblois D, Bouthillier J, Marceau F (1988) Effect of glucocorticoids, monokines and growth factors on the spontaneously developing response of the rabbit isolated aorta to des-Arg9-bradykinin. Br J Pharmacol 93: 969–977
Ellis EF, Heizer ML, Hambrecht GSet al (1987) Inhibition of bradykinin and kallikrein induced cerebral arteriolar dilation by a specific bradykinin antagonist. Stroke 18: 792–795
Ellis EF, Chao J, Heizer ML (1989) Brain kininogen following experimental brain injury: evidence for a second event. J Neurosurg 71: 437–442
Francel PC, Dawson G (1986) Bradykinin induces a rapid release of inositol triphosphate from a neuroblastoma hybrid cell line NCB-20 that is not antagonized by enkephalin. Biochem Biophs Res Commun 135: 507–514
Greenfield JG (1939) The histology of cerebral oedema associated with intracranial tumours. Brain 62: 129–1152
Hamprecht B (1984) Cell culture as models for studying neural functions. Prog Neuropsychopharmacol Biol Psychiat 8: 481–486
Hardebo JE, Kahrstom J, Owman Cet al (1987) Vasomotor effects of neurotransmitters and neuromodulators on isolated human pial vessels. J Cerebral Blood Flow Metab 7: 612–618
Hatashita S, Hoff JT (1988) Biomechanics of brain edema in acute cerebral ischemia in the cat. Stroke 19: 91–97
Huston JP, Holzhauer MS (1988) Behaviour and electrophysiological effects of intracerbrally applied neuropeptides with special attention to DC slow wave potential changes. Ann N Y Acad Sci 525: 375–390
Kariya K, Iwaki H, Ihda Met al (1981) Central action of bradykinin; Electroencephalogram of bradykinin and its degradation system in rat brain. Jpn J Pharmacol 31: 261–267
Kontos HA, Wei EP, Povlishock JTet al (1984) Oxygen radicals mediate the cerebral arteriolar dilation of arachidonate and bradykinin. Circ Res 55: 295–303
Levy WJ, McCaffrey M, York DMet al (1984) Motor evoked potentials from transcranial stimulation of the motor coretex in cats. Neurosurgery 15: 214–227
Lewis GD, Campbell WB, Johnson AR (1986) Inhibition of prostaglandin synthesis by glucocorticoids in human endothelial cells. Endocrinology 119: 62–69
Maier-Hauff K, Baethmann AJ, Lange Met al (1984) The kallikrein-kinin system as a mediator in vasogenic brain edema Part II: Studies on kinin formation in focal and perifocal brain tissue. J Neurosurg 61: 97–106
Marmarou A, Shulman K, LaMorgese J (1975) Compartmental analysis of compliance and outflow resistance of the cerebrospinal fluid system. J Neurosurg 48: 523–534
Marmarou A, Takagi H, Shulman K (1980) Biomechanics of brain edema and effects on local blood flow. Adv Neurol 28: 345–358
Marmarou A, Tanaķa K, Shulman K (1982) An improved gravimetric measure of cerebral edema. J Neurosurg 56: 246–253
Osugi Y, Uchida S, Imaizumi Tet al (1986) Bradykinin induced intracellular Ca2+ elevation in neuroblastoma × glioma hybrid NG108-15 cells: relationship to the action of inositol phospholipid metabolites. Brain Res 379: 84–89
Pirola CJ, Balda MS, Alvarez Aet al (1986) Interaction between acetylcholine and bradykinin in the lateral septal area of rat brain: Involvement of muscarinic receptors in the cardiovascular response. Neuropharmacol 25: 1387–1393
Poliakova AG (1972) Origin of early component of thye evoked response in the association cortex of the cat. Electroencephal Clin Neurophysiol 32: 129–138
Raymond JJ, Robertson DM, Dinsdale HB (1986) Pharmacological modification of bradykinin induced breakdown of the blood brain barrier. Canad J Neurol Sci 13: 214–220
Regoli D (1986) Kinins, receptors and antagonists. Adv Exp Med Biol 198: 549–558
Regoli D, Barabe J (1980) The pharmacology of bradykinin and related kinins. Pharm Rev 32: 1–47
Reiser G, Hamprecht B (1982) Bradykinin induces hyperpolerization in rat glioma cells and neuroblastoma×glioma hybrid cells. Brain Res 239: 191–199
Reiser G, Hamprecht B (1985) Bradykinin causes a transient rise of intracellular Ca2+ activity in cultured neuronal cells. Pflügers Arch 405: 260–264
Reiser G, Walter U, Hamprecht B (1984) Bradykinin regulates the level of guanosine 3′,5′ cyclic monophosphate in neural cell lines. Brain Res 290: 367–371
Seitz RJ, Wechsler W (1987) Immunohistochemical demonstration of serum proteins in human cerebral gliomas. Acta Neuropathol (Berl) 73: 145–152
Stewart PA, Hayakawa K, Farrel CIet al (1987) Quantitative study of microvessel ultrastructure in human peritumoural brain tissue. J Neurosurg 67: 697–705
Szymas J, Hossmann KA (1984) Immunofluorescent investigation of extravasated serum proteins in human brain tumour and adjacent structures. Acta Neurochir (Wien) 71: 229–241
Unterberg A, Baethmann A (1984) The kallikrein-kinin system as a mediator in vasogenic brain edema. Part 1: Cerebral exposure to bradykinin and plasma. J Neurosurg 61: 87–96
Unterberg A, Wahl M, Baethmann A (1984) Effects of bradykinin on permeability and diameter of pial vessels in vivo. J Cerebral Blood Flow Metab 4: 574–585
Unterberg A, Dautermann C, Baethmann Aet al (1986) The kallikrein-kinin system as mediators in vasogenic brain edema; Part 3, Inhibition of kallikrein-kinin system in traumatic brain swelling. J Neurosurg 64: 269–276
Wahl M, Young AR, Edvinsson Let al (1983) Effects of bradykinin on pial arteries and arterioles in vitro and in situ. J Cerebral Blood Flow Metab 3: 231–237
Wahl M, Lauritzen M, Schilling L (1987) Change of cerebrovascular reactivity after cortical spreading depression in cats and rats. Brain Res 411: 72–80
Wahl M, Unterberg A, Baethmann A (1988) Mediators of blood brain barrier dysfunction and formation of vasogenic brain oedema. J Cerebral Blood Flow Metab 8: 621–634
Walstra G, Takagi H, Marmarou Aet al (1980) The time course of brain tissue compliance and resistance in a controlled model of brain edema. In: Shulman K, Marmarou A, Miller JD, Becker D, Hochwald G, Brock M (eds) Intracranial pressure IV. Springer, Berlin, Heidelberg New York pp 253–256
Watanabe M, Rosenblum WI (1987) In vivo studies of pial vascular permeability to sodium fluorescein; absence of alterations by bradykinin, histamine, serotonin or arachidonic acid. Stroke 18: 1157–1159
Whittle IR, Piper IR, Miller JD (1991) The contribution of arachidonic acid to the etiology and pathophysiology of vasogenic brain oedema: Studies using an infusion model. Acta Neurochir (Wien) 113: 57–68
Whittle IR, Piper IR, Miller JD (1990) The contribution of secondary mediators to the etiology and pathophysiology of vasogenic brain oedema: Studies using an infusion model. Acta Neurochir [Suppl] (Wien) 51: 71–73
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Whittle, I.R., Piper, I.R. & Miller, J.D. The role of bradykinin in the etiology of vasogenic brain edema and perilesional brain dysfunction. Acta neurochir 115, 53–59 (1992). https://doi.org/10.1007/BF01400591
Issue Date:
DOI: https://doi.org/10.1007/BF01400591