Summary
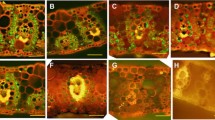
Microtubules (MTs) in cells of various tissues at different distances from the apex of the maize root exhibited different sensitivities to cold (5 °C), as judged by MT reorientation and tendency to depolymerization. Their responses seem to be related to their initial intracellular arrangements. Generally, MTs in cells which were ceasing elongation were the least sensitive during the early stages (6–24 h) of cold treatment, but during the later stages (5–7 d) MTs in most of these cells eventually depolymerized. Pericycle cells showed a unique cold response. Here the MTs were conspicuously cold-labile and quickly depolymerized near the root-tip. However, after 1 d many pericycle cells in more proximal regions had repolymerized their MTs as dense, randomly organized arrays. These persisted for the remainder of the cold treatment. A similar resistance to longterm chilling, by means of MT repolymerization, was found in cells of the root cap, quiescent centre and cells of the distal part of the former meristem. MT repolymerization in the cold may enable the apex to resume growth when more favourable (warmer) conditions return.
Similar content being viewed by others
Abbreviations
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DMSO:
-
dimethylsulfoxide
- EGTA:
-
ethylene glycol-bis(β-aminoethylether)-N,N,N′,N′-tetraacetic acid
- FITC:
-
fluorescein isothiocyanate
- IgG:
-
immunoglobulin G
- MT:
-
microtubule
- PEG:
-
polyethylene glycol
- PIPES:
-
piperazine-N,N′-bis(diethanesulfonic acid)
References
Akashi T, Shibaoka H (1987) Effects of gibberellin on the arrangement and the cold stability of cortical microtubules in epidermal cells of pea internodes. Plant Cell Physiol 28: 339–348
— — (1991) Involvement of transmembrane proteins in the association of cortical microtubules with the plasma membrane in tobacco BY-2 cells. J Cell Sci 98: 169–174
—, Izumi K, Nagano E, Enomoto M, Mizuno K, Shibaoka H (1988) Effects of propyzamide on tobacco cell microtubules in vivo and in vitro. Plant Cell Physiol 29: 1053–1062
—, Kawasaki S, Shibaoka H (1990) Stabilization of cortical microtubules by the cell wall in cultured tobacco cells. Effects of extensin on the cold-stability of cortical microtubules. Planta 182: 363–369
Åström H, Virtanen I, Raudaskoski M (1991) Cold-stability in the pollen tube cytoskeleton. Protoplasma 160: 99–107
Baluška F, Parker JS, Barlow PW (1992) Specific patterns of cortical and endoplasmic microtubules associated with cell growth and tissue differentiation in roots of maize (Zea mays L.). J Cell Sci 103: 191–200
Barlow PW, Rathfelder EL (1985) Cell division and regeneration in primary root meristems ofZea mays recovering from cold treatment. Environ Exp Bot 25: 303–314
—, Adam JS (1989 a) The response of the primary root meristem ofZea mays L. to various periods of cold. J Exp Bot 40: 81–88
— — (1989 b) Anatomical disturbances in primary roots ofZea mays following periods of cool temperature. Environ Exp Bot 29: 323–336
Bartolo ME, Carter JV (1991) Microtubules in mesophyll cells of nonacclimated and cold-acclimated spinach. Visualization and responses to freezing, low temperature, and dehydration. Plant Physiol 97: 175–181
Carter JV, Wick SM (1984) Irreversible microtubule depolymerization associated with freezing injury inAllium cepa root tip cells. Cryo Lett 5: 373–382
Chu B, Xin Z, Li PH, Carter JV (1992) Depolymerization of cortical microtubules is not a primary cause of chilling injury in corn (Zea mays L. or Black Mexican Sweet) suspension culture cells. Plant Cell Environ 15: 307–312
Clayton L, Black MC, Lloyd CW (1985) Microtubule nucleating sites in higher plant cells identified by an autoantibody against pericentriolar material. J Cell Biol 101: 319–324
Cleland R, Karlsnes AM (1967) A possible role of hydroxyprolinecontaining proteins in the cessation of cell elongation. Plant Physiol 42: 669–671
Cyr RJ, Palevitz BA (1989) Microtubule-binding proteins from carrot. I. Initial characterization and microtubule bundling. Planta 177: 245–260
Hardham AR, Gunning BES (1979) Interpolation of microtubules into cortical arrays during cell elongation and differentiation in roots ofAzolla pinnata. J Cell Sci 37: 411–442
Hensel W (1989) Tissue slices from living root caps as a model system in which to study cytodifferentiation of polar cells. Planta 177: 296–303
Hogetsu T (1986) Re-formation of microtubules inClosterium ehrenbergii Meneghini after cold-induced depolymerization. Planta 167: 437–443
Jaunin F, Hofer R-M, Pernet J-J (1985) Transport and radioactivity from tritiated abscisic acid in intact and freeze-decapped maize roots. J Plant Physiol 121: 407–415
Johnson GD, Nogueira Araujo GMC (1981) A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods 43: 349–350
Jumper BE, Lawton JR (1979) The effect of caffeine, different fixation regimes, and low temperature on microtubules in the cells of higher plants. Evidence for diversity in their response to chemical and physical treatments. Planta 145: 411–416
Kerr GP, Carter JV (1990 a) Relationship between freezing tolerance of root-tip cells and cold stability of microtubules in rye (Secale cereale L. cv. Puma). Plant Physiol 93: 77–82
— — (1990 b) Tubulin isotypes in rye roots are altered during cold acclimation. Plant Physiol 93: 83–88
Lloyd CW (1984) Towards a dynamic helical model for the influence of microtubules on wall patterns in plants. Int Rev Cytol 86: 1–51
—, Slabas AR, Powell AJ, Lowe SB (1980) Microtubules, protoplasts and plant cell shape. An immunofluorescent study. Planta 147: 500–506
Mita T, Shibaoka H (1984) Gibberellin stabilizes microtubules in onion leaf sheath cells. Protoplasma 119: 100–109
Mizuno K (1985) In vitro assembly of microtubules from tubulins of several higher plants. Cell Biol Int Rep 9: 13–21
Norenburg JL, Barrett JM (1987) Steedman's wax embedment and de-embedment for combined light and scanning electron microscopy. J Electron Microsc Tech 6: 35–41
Rikin A, Waldman M, Richmond AE, Dovrat A (1975) Hormonal regulation of morphogenesis and cold resistance. J Exp Bot 26: 175–183
—, Blumenfeld A, Richmond AE (1976) Chilling resistance as affected by stressing environments and abscisic acid. Bot Gaz 137: 307–312
Roberts IN, Lloyd CW, Roberts K (1985) Ethylene-induced reorientations: mediation by helical arrays. Planta 164: 439–447
Sakiyama M, Shibaoka H (1990) Effects of abscisic acid on the orientation and cold stability of cortical microtubules in epicotyl cells of the dwarf pea. Protoplasma 157: 165–171
Seagull RW (1990) The effects of microtubule and microfilament disrupting agents on cytoskeletal arrays and wall deposition in developing cotton fibres. Protoplasma 159: 44–59
Wasteneys GO, Jablonsky PP, Williamson RE (1989) Assembly of purified brain tubulin at cortical and endoplasmic sites in perfused internodal cells of the algaNitella tasmanica. Cell Biol Int Rep 13: 513–527
Williamson RE (1991) Orientation of cortical microtubules in interphase plant cells. Int Rev Cytol 129: 135–206
Wilms FHA, Derksen J (1988) Reorganization of cortical microtubules during cell differentiation in tobacco expiants. Protoplasma 146: 127–132
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Baluška, F., Parker, J.S. & Barlow, P.W. The microtubular cytoskeleton in cells of cold-treated roots of maize (Zea mays L.) shows tissue-specific responses. Protoplasma 172, 84–96 (1993). https://doi.org/10.1007/BF01379366
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01379366