Summary
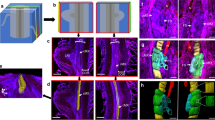
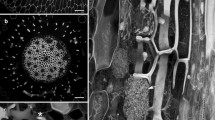
Polyclonal antibodies specific to (1→3)-β-glucose were used to localize callose around stylets ofCriconemella xenoplax in parasitized cortical cells in root explants of carnation, crimson clover, and tomato. The nematode's stylet was inserted 5–6 μm through the wall of the parasitized cell without piercing the plasma membrane, which became invaginated around the stylet tip. A layer of electron-transparent callose was localized by immunogold labelling between the invaginated plasma membrane and the inserted stylet, except at the stylet orifice. The callose was continuous with the inner surface of the wall of the parasitized cell around the site where the stylet penetrated. When the parasitized cell was located in the second layer of the cortex, the nematode's stylet first passed through a subepidermal cortical cell. The integrity of the plasma membrane of the transected cell was maintained and callose was deposited around the portion of the nematode's stylet that traversed the cell. We suggest that callose deposition around nematode stylets in parasitized cells is a common wound response elicited when plant-parasitic nematodes feed from cells.
Similar content being viewed by others
References
Aist JR (1976) Papillae and related wound plugs of plant cells. Annu Rev Phytopathol 14: 145–163
—, Bushnell WR (1991) Invasion of plants by powdery mildew fungi, and cellular mechanisms of resistance. In: Cole GT, Hoch HC (eds) The fungal spore and disease initiation in plants and animals. Plenum, New York, pp 321–345
Endo BY (1991) Ultrastructure of initial responses of susceptible and resistant soybean roots to infection byHeterodera glycines. Rev Nematol 14: 73–94
Goodman RN, Kiraly Z, Wood KR (1986) The biochemistry and physiology of plant disease. University of Missouri Press, Columbia
Harder DE, Chong J (1984) Structure and physiology of haustoria. In: Bushnell WR, Roelfs AP (eds) The cereal rusts, vol 1. Academic Press, New York, pp 431–476
Hussey RS, Mims CW, Westcott SW III (1992) Ultrastructure of root cortical cells parasitized by the ring nematodeCriconemella xenoplax. Protoplasma 167: 55–65
Kauss H (1990) Role of the plasma membrane in host-pathogen interactions. In: Larsson C, Møller IM (eds) The plant plasma membrane. Springer, Berlin Heidelberg New York Tokyo, pp 320–350
Kovats K, Binder A, Hohl HR (1991) Cytology of induced systemic resistance of cucumber toColletotrichum lagenarium. Planta 183: 484–490
Meikle PJ, Bonig I, Hoogenraad NJ, Clarke AE, Stone BA (1991) The location of (1→3)-β-glucans in the walls of pollen tubes ofNicotiana alata using a (1→3)-β-glucan-specific monoclonal antibody. Planta 185: 1–8
Nims RC, Halliwell RS, Rosberg DW (1967) Wound healing in cultured tobacco cells following microinjection. Protoplasma 64: 305–314
Northcote DH, Davey R, Lay J (1989) Use of antisera to localize callose, xylan and arabinogalactan in the cell-plate, primary and secondary walls of plant cells. Planta 178: 353–366
Rebois RV (1980) Ultrastructure of a feeding peg and tube associated withRotylenchulus reniformis in cotton. Nematologica 26: 396–405
Schuerger AC, McClure MA (1983) Ultrastructural changes induced byScutellonema brachyurum in potato roots. Phytopathology 73: 70–81
Skou JP, Jorgensen JH, Lilhot U (1984) Comparative studies of callose formation in powdery mildew compatible and incompatible barley. Phytopathol Z 109: 147–168
Smith MM, McCully ME (1978) A critical evaluation of the specificity of aniline blue induced fluorescence. Protoplasma 95: 229–254
Van der Woude C, Lembi A, Morré DJ, Kindinger JL, Ordin L (1974) β-Glucan synthetases of plasma membrane and Golgi apparatus from onion stem. Plant Physiol 54: 333–340
Westcott SW, Hussey RS (1992) Feeding behavior ofCriconemella xenoplax in monoxenic cultures. Phytopathology 82: 936–940
Wyss U, Stender C, Lehmann H (1984) Ultrastructure of feeding sites of the cyst nematodeHeterodera schachtii Schmidt in roots of susceptible and resistantRaphanus sativus L. var.oleiformis cultivars. Physiol Plant Pathol 25: 21–37
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hussey, R.S., Mims, C.W. & Westcott, S.W. Immunocytochemical localization of callose in root cortical cells parasitized by the ring nematodeCriconemella xenoplax . Protoplasma 171, 1–6 (1992). https://doi.org/10.1007/BF01379274
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01379274