Summary
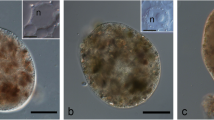
Polyphosphate granules are precipitated in the vacuoles of the ectomycorrhizal fungusPisolithus tinctorius (Pers.) Coker & Couch by various treatments, including conventional specimen preparation. Granules are not produced by glutaraldehyde fixation but appear at early stages of ethanol dehydration and are visible with Nomarski DIC microscopy. They show γ-metachromasy with toluidine blue O at low pH, are extracted by cold trichloroacetic acid and contain phosphorus and calcium as demonstrated by X-ray microanalysis. The granules are surrounded by electron-lucent areas that do not contain these elements at detectable levels. In contrast, vacuoles of freeze-substituted hyphae contain evenly dispersed flocculent material. Phosphorus and potassium are distributed more or less uniformly throughout, but calcium is not detected. This indicates that polyphosphate is present in the vacuole of living hyphae in soluble form and is precipitated to form granules by various treatments. It is thought that granules form when membranes, including the tonoplast, become leaky and there is an influx of precipitating ions such as calcium.
Similar content being viewed by others
Abbreviations
- DIC:
-
differential interference contrast
- GMA:
-
glycol methacrylate
- MMN:
-
modified Melin Norkrans
- NMR:
-
nuclear magnetic resonance
- Pi :
-
inorganic phosphate
- STEM:
-
scanning transmission electron microscope
References
Ashford AE, Ling-Lee M, Chilvers GA (1975) Polyphosphate in eucalypt mycorrhizas: a cytochemical demonstration. New Phytol 74: 477–453
—, Peterson RL, Dwarte D, Chilvers GA (1986) Polyphosphate granules in eucalypt mycorrhizas: determination by energy dispersive X-ray microanalysis. Can J Bot 64: 677–687
Bieleski RL (1973) Phosphate pools, phosphate transport, and phosphate availability. Annu Rev Plant Physiol 24: 225–252
Brunk UT, Ericsson JLE (1973) The demonstration of acid phosphatase activity in in vitro cultured tissue cells. Studies on the significance of fixation, tonicity and permeability. In: Stoward PJ (ed) Fixation in histochemistry. Chapman and Hall, London, pp 121–135
Callow JA, Capaccio LCM, Parish G, Tinker PB (1978) Detection and estimation of polyphosphate in vesicular-arbuscular mycorrhizas. New Phytol 80: 125–134
Canny MJ, McCully ME (1986) Locating water-soluble vital stains in plant tissues by freeze-substitution and resin-embedding. J Microsc 142: 63–70
Chilvers GA, Lapeyrie FF, Douglass PA (1985) A contrast between oomycetes and other taxa of mycelial fungi in regard to metachromatic granule formation. New Phytol 99: 203–210
Cox G, Moran KJ, Sanders F, Nockolds C, Tinker PB (1980) Translocation and transfer of nutrients in vesicular-arbuscular mycorrhizas. III. Polyphosphate granules and phosphorus translocation. New Phytol 84: 649–659
—, Sanders FE, Tinker PB, Wild JA (1975) Ultrastructural evidence relating to host-endophyte transfer in a vesicular-arbuscular mycorrhiza. In: Sanders FE, Mosse B, Tinker PB (eds) Endomycorrhizas. Academic Press, London, pp 297–312
Crang RE (1980) Polyphosphate bodies inAureobasidium pullulans. Micron 11: 3–4
Daddow LYM (1983) A double lead stain method for enhancing contrast of ultrathin sections in electron microscopy: a modified multiple staining technique. J Microsc 129: 147–153
Davey DF (1973) The effect of fixative tonicity on the myosin filament lattice volume of frog muscle fixed following exposure to normal or hypertonic Ringer. In: Stoward PJ (ed) Fixation in histochemistry. Chapman and Hall, London, pp 103–120
Delaporte B (1939) Sur les acides nucléiques des levures et leur localisation. Rev Gén Bot 51: 449–482
Denny HJ, Wilkins DA (1987) Zinc tolerance inBetula spp. IV. The mechanism of ectomycorrhizal amelioration of zinc toxicity. New Phytol 106: 545–553
Doonan BB, Crang RE, Jensen TE, Baxter M (1979) In situ X-ray energy dispersive microanalysis of polyphosphate bodies inAureobasidium pullulans. J Ultrastruct Res 69: 232–238
Fitzgerald MA, Allaway WG (1991) Apoplastic and symplastic pathways in the leaf of the grey mangroveAvicennia marina. New Phytol 119: 217–226
Gianinazzi-Pearson V, Gianinazzi S (1989) Phosphorus metabolism by mycorrhizas. In: Boddy L, Marchant R, Read DJ (eds) Nitrogen phosphorus and sulphur utilization by fungi. Cambridge University Press, Cambridge, pp 226–241
Grellier B, Strullu DG, Martin F, Renaudin S (1989) Synthesis in vitro, microanalysis and31P-NMR study of metachromatic granules in birch mycorrhizas. New Phytol 112: 49–54
Grenville DJ, Peterson RL, Ashford AE (1986) Synthesis in growth pouches of mycorrhizae betweenEucalyptus pilularis and several strains ofPisolithus tinctorius. Aust J Bot 34: 95–102
Harley JL, Smith SE (1983) Mycorrhizal symbiosis. Academic Press, London
Jacobson L, Halmann M, Yariv J (1982) The molecular composition of the volutin granule of yeast. Biochem J 201: 473–479
Jennings DH (1987) Translocation of solutes in fungi. New Phytol 62: 215–243
— (1989) Nitrogen and phosphorus metabolism in fungi. In: Boddy L, Marchant R, Read DJ (eds) Nitrogen phosphorus and sulphur utilization by fungi. Cambridge University Press, Cambridge, pp 1–31
Keck K, Stich H (1957) The widespread occurrence of polyphosphate in lower plants. Ann Bot 21: 611–619
Klionsky DJ, Herman PK, Emr SD (1990) The fungal vacuole: composition, function, and biogenesis. Microbiol Rev 54: 266–292
Kulaev IS, Vagabov M (1983) Polyphosphate metabolism in microorganisms. Adv Microbiol Physiol 24: 83–158
Lapeyrie FF, Chilvers GA, Douglass PA (1984) Formation of metachromatic granules following phosphate uptake by mycelial hyphae of an ectomycorrhizal fungus. New Phytol 98: 345–360
Marshall AT (1980) Freeze-substitution as a preparation technique for biological X-ray microanalysis. Scann Electron Microsc 2: 395–408
Martin F, Canet D, Rolin D, Marchai J-P, Larher F (1983) Phosphorus-31 nuclear magnetic resonance study of polyphosphate metabolism in intact ectomycorrhizal fungi. Plant Soil 71: 469–476
—, Marchal J-P, Timinski A, Canet D (1985) The metabolism and physical state of polyphosphates in ectomycorrhizal fungi. A31P nuclear magnetic resonance study. New Phytol 101: 275–290
Marx DH (1969) The influence of ectotrophic mycorrhizal fungi on the resistance of pine roots to pathogenic infections. 1. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria. Phytopathology 59: 153–163
Miller JJ (1984) In vitro experiments concerning the state of polyphosphate in the yeast vacuole. Can J Microbiol 30: 236–246
Morgan AJ, Davies TW, Erasmus DA (1978) Specimen preparation. In: Erasmus DA (ed) Electron probe microanalysis in biology. Chapman and Hall, London, pp 94–147
Nakai Y (1976) Fine structure of shiitake,Lentinus edodes (Berk.) Sing. V. Intercellular inclusions in germinating basidiospores induced by glutaraldehyde fixation. Rep Tottori Mycol Inst (Japan) 14: 91–94
Orlovich DA, Ashford AE, Cox GC (1989) A reassessment of polyphosphate granule composition in the ectomycorrhizal fungusPisolithus tinctorius. Aust J Plant Physiol 16: 107–115
— — —, Moore AEP (1990) Freeze-substitution and X-ray microanalysis of polyphosphate granules in the mycorrhizal fungusPisolithus tinctorius (Pers.) Coker & Couch. Endocytobiology 4: 139–143
Pallaghy CK (1973) Electron probe microanalysis of potassium and chloride in freeze-substituted leaf sections ofZea mays. Aust J Biol Sci 26: 1015–1034
Reynolds ES (1963) The use of lead citrate at high pH as an electronopaque stain in electron microscopy. J Cell Biol 17: 208–210
Salema R, Brandão I (1973) The use of PIPES buffer in the fixation of plant cells for electron microscopy. J Submicrosc Cytol 5: 79–96
Shepherd VA, Orlovich DA, Ashford AE (1993) A dynamic continuum of pleiomorphic tubules and vacuoles in growing hyphae of a fungus. J Cell Sci 104 (in press)
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26: 31–43
Straker CJ, Mitchell DT (1985) The characterization and estimation of polyphosphates in endomycorrhizas of the Ericaceae. New Phytol 99: 431–440
Strullu DG, Grellier B, Garrec JP, McCready CC, Harley JL (1986) Effects of monovalent and divalent cations on phosphate absorption by beech mycorrhizas. New Phytol 103: 403–416
Thompson W, Brownlee C, Jennings DH, Mortimer AM (1987) Localized, cold-induced inhibition of translocation in mycelia and strands ofSerpula lacrimans. J Exp Bot 38: 889–899
Tijssen JPF, van Steveninck J, de Bruijn WC (1985) Cytochemical staining of a yeast polyphosphate fraction, localized outside the plasma membrane. Protoplasma 125: 124–128
van Noorden S, Polak JM (1983) Immunocytochemistry today. Techniques and practice. In: Polak JM, van Noorden S (eds) Immunocytochemistry: practical applications in pathology and biology. John Wright and Sons, Bristol, pp 11–42
Väre H (1990) Aluminium polyphosphate in the ectomycorrhizal fungusSuillus variegatus (Fr.) O. Kunze as revealed by energy dispersive spectroscopy. New Phytol 116: 663–668
Walker GD, Powell CL (1979) Vesicular-arbuscular mycorrhizas in white clover: a scanning electron microscope and X-ray microanalytical study. NZJ Bot 17: 55–59
Wiemken A, Schellenberg M, Urech K (1979) Vacuoles: the sole compartments of digestive enzymes in yeast (Saccharomyces cerevisiae). Arch Microbiol 123: 23–35
Yanagita T (1964) Germinating conidiospores ofAspergillus niger. In: Zeuthen E (ed) Synchrony in cell division and growth. Interscience Publishers, New York, pp 391–420
Young N, Bullock S, Orlovich DA, Ashford AE (1993) Association of polyphosphate with protein in freeze-substituted sclerotia ofSclerotinia minor. Protoplasma (in press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Orlovich, D.A., Ashford, A.E. Polyphosphate granules are an artefact of specimen preparation in the ectomycorrhizal fungusPisolithus tinctorius . Protoplasma 173, 91–102 (1993). https://doi.org/10.1007/BF01378998
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01378998