Summary
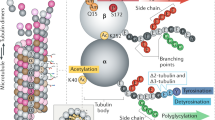
To assay the functional significance of the multiple but closely related α- and β-tubulin polypeptides (termed isotypes) that are expressed in mammalian cells, we have generated a number of sera that uniquely discriminate among these isotypes. These sera have been used to demonstrate that there is no subcellular sorting of either α- or β-tubulin isotypes among microtubules of diverse function, either in cells growing in culture or in tissues consisting of cell types that contain specialized kinds of microtubule. In spite of this failure to segregate between functionally distinct kinds of microtubule, the fact that isotype-specific amino acid sequences have been strictly conserved over extensive periods of evolutionary time argues persuasively for a functional role for the different tubulin gene products. One possibility is that they are required for specific interactions with microtubule associated proteins (MAPs), and that tubulin isotypes have coevolved with different cell type-specific MAPs with which they must interact. We have tested this hypothesis by examining the distribution of β-tubulin isotypes in mammalian cerebellum in relationship to the known patterns of expression of a number of MAPs, and find that these patterns correlate in the case of Mβ 2 and MAP 3, and Mβ 6 and MAP 1 a. These data, plus emerging data based on a structural analysis of tau, MAP 1 b and MAP 2 obtained via sequence determination of cloned cDNAs, are discussed in terms of the possible functional significance of tubulin isotype/MAP interactionsin vivo.
Similar content being viewed by others
References
Adachi Y, Toda T, Niwa O, Yanagida M (1986) Differential expression of essential and non-essential α-tubulin genes inSchizosaccaromyces pombe. Mol Cell Biol 6: 2168–2178
Aletta JM, Lewis SA, Cowan NJ, Greene LA (1988) Nerve growth factor regulates both the phosphorylation and steady state levels of microtubule associated protein 1.2 (MAP 1.2). J Cell Biol 106: 1573–1582
Argarana CE, Barra HS, Caputto R (1978) Release of [14C]-tyrosine from tubulinyl-[14C]-tyrosine by brain extract. Separation of a carboxypeptidase from tubulin tyrosine ligase. Mol Cell Biochem 19: 17–22
Barra HS, Arce CA, Rodriguez JA, Caputto R (1974) Some common properties of the protein that incorporates tyrosine as a single unit into microtubule proteins. Biochem Biophys Res Commun 60: 1384–1390
Binder LI, Frankfurter A, Rebhun LI (1985) The distribution of tau in the mammalian central nervous system. J Cell Biol 101: 1371–1378
Bloom GS, Luca FD, Vallee RB (1984) Widespread distribution of the major component of MAP 1 (microtubule associated protein 1) in the central nervous system. J Cell Biol 98: 331–340
Bond JF, Fridovich-Keil JL, Pillus L, Mulligan RC, Solomon F (1986). A chicken-yeast chimeric β-tubulin protein is incorporated into mouse microtubulesin vivo. Cell 44: 461–468
Burgoyne RD,Cambray-Deakin, MA,Lewis, SA,Sarkar, S,Cowan, NJ (1988) Differential distribution of β-tubulin isotypes in cerebellum. EMBO J, in press
Cleveland DW, Hwo SY, Kirschner MW (1977) Purification of tau, a microtubule associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol 116: 207–225
Cowan NJ, Dobner PR, Fuchs EV, Cleveland DW (1983) Expression of human α-tubulin genes: interspecies conservation of 3′ untranslated regions. Mol Cell Biol 3: 1738–1745
Lewis SA,Sarkar S,Gu W (1987) Functional versatility of mammalian β-tubulin isotypes. In:Maccioni R, Arechaga J (eds) The cytoskeleton in cell differentiation and development. ICSU Press, pp 157–166
Drubin DG, Caput D, Kirschner MW (1984) Studies on the expression of microtubule associated protein tau during mouse brain development with newly isolated complementary DNA probes. J Cell Biol 98: 1090–1097
Elliot EM, Okayama H, Savangi F, Henderson G, Ling V (1985) Differential expression of three α-tubulin genes in Chinese Hamster ovary cells. Mol Cell Biol 5: 236–241
Farmer SR, Bond JF, Robinson GS, Mbandgollo D, Fenton MJ, Berkowitz EH (1984) Differential expression of the rat β-tubulin multigene family. In:Borisy GG, Cleveland DW, Murphy DB (eds) Molecular biology of the cytoskeleton. Cold Spring Harbor Publications, NY, pp 333–342
Fuller MT, Caulton JH, Hutchens JA, Kaufman TC, Raff EC (1987) Genetic analysis of microtubule structure: A β-tubulin mutation causes the formation of aberrant microtubulesin vivo andin vitro. J Cell Biol 104: 385–394
Gu W,Lewis SA,Cowan NJ (1988) Generation of antisera that discriminate among mammalian α-tubulins: Introduction of specialized isotypes into cultured cells results in their coassembly without disruption of normal microtubule function. J Cell Biol, in press
Hall JL, Dudley L, Dobner PR, Lewis SA, Cowan NJ (1983) Identification of two human β-tubulin isotypes. Mol Cell Biol 3: 854–862
Huber G, Matus A (1984) Differences in the cellular distributions of two microtubule associated proteins, MAP 1 and MAP 2, in rat brain. J Neurosci 4: 151–160
Kemphues KJ, Kaufman TC, Raff RA, Raff EC (1982) The testisspecific β-tubulin subunit inDrosophila melanogaster has multiple functions in spermatogenesis. Cell 31: 655–670
L'Hernault SW, Rosenbaum JL (1983)Chlamydomonas α-tubulin is post-translationally modified in the flagella during flagellar assembly. J Cell Biol 97: 258–269
Lee G, Cowan NJ, Kirschner MW (1988) The primary structure and heterogeneity of tau protein from mouse brain. Science 239: 285–288
Lee MG-S, Loomis C, Cowan NJ (1984) Sequence of an expressed human β-tubulin gene containing tenAlu family members.Nucleic Acids Res 12: 5823–5836
—,Lewis SA, Wilde CD, Cowan NJ (1983) Evolutionary history of a multigene family: an expressed human β-tubulin gene and three processed pseudogenes. Cell 33: 477–487
Lewis SA, Lee MG-S, Cowan NJ (1985 a) Five mouse tubulin isotypes and their regulated expression during development. J Cell Biol 101: 852–861
—,Gilmartin ML, Hall JL, Cowan NJ (1985 b) Three expressed sequences within the human β-tubulin multigene family each define a distinct isotype. J Mol Biol 182: 11–20
—,Sherline P, Cowan NJ (1986) A cloned cDNA encoding MAP 1 detects a single copy gene in mouse and a brain-abundant RNA whose level decreases during development. J Cell Biol 102: 2106–2114
—,Villasante A, Sherline P, Cowan NJ (1986) Brain-specific expression of MAP 2 detected using a cloned cDNA prove. J Cell Biol 102: 2098–2105
—,Cowan NJ (1987) Free intermingling of mammalian β-tubulin isotypes among functionally distinct microtubules. Cell 49: 59–548
—Cowan NJ (1988) Complex regulation and functional versatility of α- and β-tubulin isotypes during the differentiation of testis and muscle cells. J Cell Biol, in press
Lopata MA, Cleveland DW (1987)In vivo microtubules are copolymers of available β-tubulin isotypes: localization of each of six vertebrate β-tubulin isotypes using polyclonal antibodies elicited by synthetic peptide antigens. J Cell Biol 105: 1707–1720
May GS, Gambino J, Weatherbee JA, Morris NR (1985) Identification and functional analysis of β-tubulin genes by site-specific integration inAspergillus nidulans. J Cell Biol 101: 712–719
Parysek LM, Asnes CF, Olmsted JB (1984) MAP 4: occurrence in mouse tissue. J Cell Biol 99: 1309–1315
Raybin D, Flavin M (1977) Enzyme which specifically adds tyrosine to the α-chain of tubulin. Biochemistry 16: 2189–2194
Shatz PJ, Pillus L, Grisafi P, Solomon F, Botstein D (1986 a) Two functional α-tubulin genes of the yeastSaccharomyces cerevisiae encode divergent proteins. Mol Cell Biol 6: 3711–3721
—,Solomon F, Botstein D (1986 b) Genetically essential and nonessential α-tubulin genes specify functionally interchangeable proteins. Mol Cell Biol 6: 3722–3733
Sullivan KF, Cleveland DW (1986) Identification of conserved isotype-defining variable region sequences for four vertebrate β-tubulin peptide classes. Proc Natl Acad Sci USA 83: 4327–4331
Villasante A, Wang D, Dobner P, Dolph P, Lewis SA, Cowan NJ (1986) Six mouse α-tubulin mRNAs encode five distinct tubulin isotypes: testis-specific expression of two sister genes. Mol Cell Biol 6: 2409–2419
Wang D, Villasante A, Lewis SA, Cowan NJ (1986) The mammalian β-tubulin repertoire: hematopoietic expression of a novel β-tubulin isotype. J Cell Biol 103: 1903–1910
Weatherbee JA, May GS, Gambino J, Morris NR (1985) Involvement of a particular species of β-tubulin (B 3) in conidial development inAspergillus nidulans. J Cell Biol 101: 712–719
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cowan, N.J., Lewis, S.A., Gu, W. et al. Tubulin isotypes and their interaction with microtubule associated proteins. Protoplasma 145, 106–111 (1988). https://doi.org/10.1007/BF01349346
Issue Date:
DOI: https://doi.org/10.1007/BF01349346