Summary
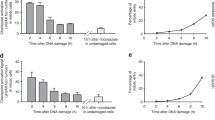
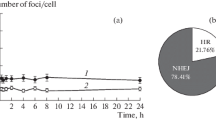
The ability of synchronized Ehrlich ascites tumour cells to repair PLD was measured by introducing delays in their progression through the cell cycle either in the same phase as that where the irradiation was given or in a subsequent phase. Cells were incubated for this purpose either in balanced salt solution which nonspecifically delayed progression in all cell cycle phases or with 0.5 µg/ml aphidicolin which delayed cells in S-phase. Cells which had been delayed in their progression through the cell cycle were able to repair PLD irrespective of the phase at which they were held. In cases where the delay in the progression through the cell cycle was introduced in a phase subsequent to that of the exposure to irradiation, repair of PLD was observed only if the cells had not passed the G1/S-border or mitosis. Based on these results, the importance of G1/S-border and mitosis in the fixation of PLD is suggested.
Similar content being viewed by others
References
Böhmer RM (1979) Flow cytometric cell cycle analysis using the quenching of 33258 Hoechst fluorescence by bromodeoxyuridine incorporation. Cell Tissue Kinet 12: 101–110
De Brabander MJ, van de Veire RML, Aerts FEM, Borgers M, Janssen PAJ (1976) The effects of methyl (5-(2-thienylcarbonyl-1H-benzimidazol-2y1)carbamate (R 17934; NSC 238159), a new synthetic antitumoral drug interfering with microtubules, on mammalian cells cultured in vitro. Cancer Res 36: 305
Frankenberg D (1969) A ferrous sulphate dosemeter independent of photon energy in the range from 25 keV up to 50 MeV. Phys Med Biol 14: 597–605
Hahn GM, Little JB (1972) Plateau phase cultures of mammalian cells. An in vitro model for human cancer. Curr Top Radial Res Q 8: 39–83
Ikegami S, Taguchi T, Ohashi M, Oguro M, Nagano H, Mano Y (1978) Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymeraseα. Nature 275: 548–460
Iliakis G (1980) Repair of potentially lethal damage in unfed plateau phase cultures of Ehrlich ascites tumour cells. I. Suspension cultures. Int J Radiat Biol 37: 365–372
Iliakis G (1980) Effects ofβ-arabinofuranosyladenine on the growth and repair of potentially lethal damage in Ehrlich ascites tumour cells. Radiat Res 83: 537–552
Iliakis G (1981) Characterization and properties of repair of potentially lethal damage as measured with the help ofβ-arabinofuranosyladenine in plateau phase EAT-cells Radiat Res 86: 77–90
Iliakis G, Nüsse M (1982a) Aphidicolin promotes repair of potentially lethal damage in irradiated mammalian cells synchronized in S-phase. Biochem Biophys Res Commun 104: 1209–1214
Iliakis G, Nüsse M (1982b) Conditions supporting repair of potentially lethal damage cause a significant reduction of ultraviolet light-induced division delay in synchronized and plateau phase Ehrlich ascites tumour cells. Radiat Res 91: 483–506
Iliakis G, Nüsse M, Bryant P (1982) Effects of aphidicolin on cell proliferation, repair of potentially lethal damage and repair of DNA strand breaks in Ehrlich ascites tumour cells irradiated with x-rays. Int J Radiat Biol 42: 417–434
Iliakis G, Nüsse M (1983) Evidence that repair and expression of potentially lethal damage cause the variations in cell survival after x-irradiation observed through the cell cycle in Ehrlich ascites tumour cells. Radiat Res 95: 87–107
Iliakis G, Nüsse M (1983) Arrest of irradiated G1-, S- or G2-cells at mitosis using nocodazole promotes repair of potentially lethal damage (In preparation)
Iliakis G, Pohlit W (1979) Quantitative aspects on the repair of potentially lethal damage in mammalian cells. Int J Radiat Biol 36: 649–658
Karzel K (1965) über einen in vitro in Suspension wachsenden permanenten Stamm von Ehrlich-Aszites-Tumorzellen. Med Pharm Exp 12: 137–144
Nüsse M (1981) Cell cycle kinetics of irradiated synchronous and asynchronous tumour cells using DNA distribution analysis and BrdUrd-Hoechst 33258-technique. Cytometry 2: 70–79
Nüsse M (1982) Synchronization of mammalian cells by selection and additional chemical block studied by DNA distribution analysis and BrdUrd-Hoechst 33258-technique. Cell Tissue Kinet 15: 529–543
Phillips RA, Tolmach LJ (1966) Repair of potentially lethal damage in x-irradiated HeLa cells. Radiat Res 29: 413–432
Pohlit W, Heyder IR (1981) The shape of dose-survival curves for mammalian cells and repair of potentially lethal damage analyzed by hypertonic treatment. Radiat Res 87: 613–634
Ziewe GW, Turnbull D, Mullins JM, McIntosh JR (1980) Production of large numbers of mitotic mammalian cells by use of the reversible microtubule inhibitor nocodazole. Exp Cell Res 126: 397–405
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iliakis, G., Nüsse, M. The importance of G1/S-border and mitosis in the fixation of potentially lethal damage. Radiat Environ Biophys 22, 201–207 (1983). https://doi.org/10.1007/BF01323709
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01323709