Summary
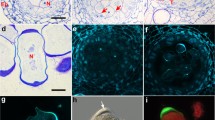
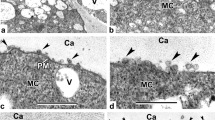
The cytokinetic apparatus in microsporogenesis lacks a preprophase band of microtubules and the selection of cytokinetic planes is dependent upon disposition of nuclei which define cytoplasmic domains via post-meiotic radial systems of microtubules. Meiotic cytokinesis was investigated in hybrid moth orchids (Phalaenopsis) exhibiting irregular patterns of cytokinesis. In these polliniate orchids, spindle orientation is imprecise, and the tetrad nuclei (therefore the microspores) may be in rhomboidal, tetrahedral or linear arrangement. The hybrid “Sabine Queen” (section Phalaenopsis) regularly undergoes simultaneous cytokinesis, as is common in orchids. The hybrid “Vista Rainbow” (section Amboinenses) produces either a complete dyad wall, a partial wall, or no wall after first nuclear division. In all cases, a first division phragmoplast is initiated in the interzonal region and expands centrifugally into the peripheral cytoplasm. Fluorescence microscopy shows that the phragmoplast consists of fusiform bundles of microtubules and Factin bisected by a non-fluorescent zone. If a cell plate fails to form, a band of organelles polarized in the equatorial region effectively divides the cell into two domains. The organelles disperse when a dyad wall is complete, but tend to remain polarized around an incomplete wall. In four-nucleate coenocytes, the usual interzonal microtubules between sister nuclei (primary) form slightly in advance of secondary arrays between non-sister nuclei. Phragmoplasts are initiated in sites defined by the post-meiotic microtubule arrays.
Similar content being viewed by others
Abbreviations
- CLSM:
-
confocal laser scanning microscope/microscopy
- DMSO:
-
dimethylsulfoxide
- FITC:
-
fluorescein isothiocyanate
- PPB:
-
preprophase band of microtubules
- TEM:
-
transmission electron microscope/microscopy
References
Albert VA, Corriveau JL, Coleman AW (1989) In situ, fluorochrome-mediated visualization of nuclear and cytoplasmic DNA: a new cytological tool for orchid pollen research. Lindleyana 4: 192–214
Bonsignore CL, Hepler PK (1985) Caffeine inhibition of cytokinesis: dynamics of cell plate formation-deformation in vivo. Protoplasma 129: 28–35
Brown RC, Lemmon BE (1987 a) Division polarity, development and configuration of microtubule arrays in bryophyte meiosis I. Meiotic prophase to metaphase I. Protoplasma 137: 84–99
— — (1987 b) Division polarity, development and configuration of microtubule arrays in bryophyte meiosis II. Anaphase I to the tetrad. Protoplasma 138: 1–10
— — (1988 a) Cytokinesis occurs at boundaries of domains delimited by nuclear-based microtubules in sporocytes ofConocephalum conicum (Bryophyta). Cell Motil Cytoskeleton 11: 139–146
— — (1988 b) Microtubules associated with simultaneous cytokinesis of coenocytic microsporocytes. Amer J Bot 75: 1848–1856
— — (1989) Minispindles and cytoplasmic domains in microsporogenesis in orchids. Protoplasma 148: 26–32
— — (1991 a) Sporogenesis in simple land plants. In: Blackmore S, Barnes SH (eds) Pollen and spores: patterns of diversification. Oxford University Press, Oxford (in press)
— — (1991 b) The cytokinetic apparatus in meiosis: control of division plane in the absence of a preprophase band of microtubules. In: Lloyd C (ed) The cytoskeletal basis of plant growth and form. Academic Press, London (in press)
Farr CH (1916) Cytokinesis of the pollen-mother-cells of certain dicotyledons. Mem NY Bot Gard 6: 253–316 +3 plates
— (1918) Cell division by furrowing inMagnolia. Amer J Bot 5: 379–395 +3 plates
Gunning BES (1982) The cytokinetic apparatus: its development and spatial regulation. In Lloyd C (ed) Cytoskeleton in plant growth and development. Academic Press, London, pp 229–292
Heslop-Harrison J (1968) Synchronous pollen mitosis and the formation of the generative cell in massulate orchids. J Cell Sci 3: 457–466
— (1971) Wall pattern formation in angiosperm microsporogenesis. Symp Soc Exp Biol 25: 277–300
Hogan CJ (1987) Microtubule patterns during meiosis in two higher plant species. Protoplasma 138: 126–136
Juel HO (1897) Die Kerntheilungen in den Pollenmutterzellen vonHemerocallis fulva und die bei denselben auftretenden Unregelmässigkeiten. Jahrb Wiss Bot 30: 205–226 +2 plates
Konta F, Tsuji M (1982) The types of pollen tetrads and their formations observed in some species in the Orchidaceae in Japan. Acta Phytotax Geobot 33: 206–217
Lloyd CW (1987) The plant cytoskeleton: the impact of fluorescence microscopy. Annu Rev Plant Physiol 38: 119–139
Paul DC, Goff CW (1973) Comparative effects of caffeine, its analogs and calcium deficiency on cytokinesis. Exp Cell Res 78: 399–413
Rodkiewicz B, Duda E (1988) Aggregations of organelles in meiotic cells of higher plants. Acta Soc Bot Pol 57: 637–654
Schliwa M, Van Berkom J (1981) Structural interaction of cytoskeletal components. J Cell Biol 98: 525–533
Sheldon JM, Hawes C (1988) The actin cytoskeleton during male meiosis inLilium. Cell Biol Int Rep 12: 471–476
Strasburger E (1880) Zellbildung und Zelltheilung. Gustav Fischer, Jena
Traas JA, Burgain S, Dumas de Vaulx R (1989) The organization of the cytoskeleton during meiosis in eggplant (Solanum melongena L.): microtubules and F-actin are both necessary for coordinated meiotic division. J Cell Sci 92: 541–550
Van Lammeren AAM, Bednara J, Willemse MTM (1989) Organization of the actin cytoskeleton during pollen development inGasteria verrucosa (Mill.) H. Duval visualized with rhodamine-phalloidin. Planta 178: 531–539
—, Keijzer CJ, Willemse MTM, Kieft H (1985) Structure and function of the microtubular cytoskeleton during pollen development inGasteria verrucosa (Mill.) H. Duval. Planta 165: 1–11
Vaughan MA, Vaughn KC (1989) DCPA causes cell plate disruption in wheat roots. Ann Bot 65: 379–388
Waterkeyn L (1962) Les parois microsporocytaires de nature callosique chezHelleborus etTradescantia. Cellule 62: 225–255 +3 plates
Yeung EC (1987) Development of pollen and accessory structures in orchids. In: Arditti J (ed) Orchid biology: reviews and perspectives, vol 4. Cornell University Press, Ithaca, pp 193–226
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brown, R.C., Lemmon, B.E. Pollen development in orchids 1. Cytoskeleton and the control of division plane in irregular patterns of cytokinesis. Protoplasma 163, 9–18 (1991). https://doi.org/10.1007/BF01323402
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01323402