Summary
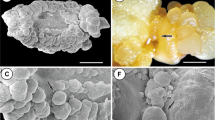
The ectomycorrhizal fungus,Paxillus involutus, produces sclerotia in culture. These can be induced to form on agar medium by exposing mycelium grown at 25°C to various temperatures between6°C and 15°C. Sclerotia formed at 10°C and above were large and covered with drops of exudate, while those formed at 6°C or 8°C were very small and did not produce an exudate. Mature sclerotia were bounded by a compact rind and contained abundant storage reserves. Histochemistry of the larger sclerotia showed large quantities of protein stored as protein bodies in the cytoplasm, lipid present as small droplets, glycogen granules stored in the cytoplasm and polyphosphate present as small granules in the cytoplasm and in the protein bodies. Energy dispersive X-ray microanalysis confirmed the presence of phosphate in the granules and was used to map its distribution throughout the sclerotium. The smaller sclerotia induced at 8°C and below on the same medium had the same basic structure and composition, but lacked the complex phenolic cell network found in large sclerotia, and had abundant extracellular polysaccharides. The rind was not well developed and these small sclerotia are interpreted to have been arrested at an early stage of development.
Similar content being viewed by others
References
Antibus RK (1989) Formation and structure of sclerotia and sclerotium-specific proteins inHygrophoropsis aurantiaca. Mycologia 81: 905–913
Ashford AE, Ling Lee M, Chilvers GA (1975) Polyphosphate in eucalypt mycorrhizas: a cytochemical demonstration. New Phytol 74: 447–453
—, Peterson RL, Dwarte D, Chilvers GA (1986) Polyphosphate granules in eucalypt mycorrhizas: determination by energy dispersive X-ray microanalysis. Can J Bot 64: 677–687
Backhouse D, Stewart A (1987) Anatomy and histochemistry of resting and germinating sclerotia ofSclerotium cepivorum. Trans Br Mycol Soc 89: 561–567
— — (1988) Large sclerotia ofSclerotium cepivorum. Trans Br Mycol Soc 91: 343–346
—, Willetts HJ (1984) A histochemical study of sclerotia ofBotrytis cinerea andBotrytis fabae. Can J Microbiol 30: 171–178
— — (1985) Histochemical changes during conidiogenic germination of sclerotia ofBotrytis cinerea. Can J Microbiol 31: 282–286
Bewley JD, Black M (1978) Physiology and biochemistry of seeds in relation to germination. Springer, Berlin Heidelberg New York
O'Brien TP, McCully ME (1981) The study of plant structure: principles and selected methods. Termarcarphi, Sydney
Baker DD, Schwintzer CR (1990) Introduction. In: Schwintzer CR, Tjepkema JD (eds) The biology ofFrankia and actinorhizal plants. Academic Press, San Diego, pp 1–13
Becking JH (1975) Root nodules in nonlegumes. In: Torrey JG, Clarkson DT (eds) Development and function of roots. Academic Press, London, pp 508–566
O'Brien TP, McCully ME (1981) The study of plant structure: principles and selected methods. Termarcarphi Pty, Melbourne
Rensselaer MV, McMinn HE (1942) Ceanothus. Gillick Press, Berkeley, CA
Sexton R, Burdon JN, Reid SG, Durbin ML, Lewis LN (1984) Cell wall breakdown and abscission. In: Dugger WR, Bartinicki-Garcia S (eds) Structure, function and biosynthesis of plant cell walls. Waverly Press, Baltimore, MD, pp 195–221
Takhtajan A, Jeffrey C (1969) Flowering plants: origin and dispersal. Smithsonian Institution Press, Washington, DC
Thomas BA, Spicer RA (1987) The evolution of modern vegetation. In: Dudley TR (ed) The evolution and palaeobiology of land plants. Dioscorides Press, Portland, OR, pp 267–276
Wiatr SM (1978) Physiology and ultrastructure of petal abscission in western blue flax (Linum lewisii). PhD dissertation, University of California, Davis, CA
Berry AM, Sunell LA (1990) The infection process and nodule development. In: Schwintzer CR, Tjepkema JD (eds) The biology ofFrankia and actinorhizal plants. Academic Press, San Diego, pp 61–81
Steel RGD, Torrie JH (1980) Principles and procedures of statistics, a biometrical approach. McGraw-Hill, New York
Byers B (1981) Cytology of the yeast life cycle. In: Strathern JN, Jones EW, Broach JR (eds) The molecular biology of the yeastSaccharomyces: life cycle and inheritance. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp 59–96
Gunning BES (1982) The cytokinetic apparatus: its development and spatial regulation. In: Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 229–294
Cherry JH, Heuss-LaRosa K, Meyer RR (1989) Adaptation of thermotolerance in cowpea suspension cultures. In: Cherry JH (ed) Environmental stress in plants: biochemical and physiological mechanism. Springer, Berlin Heidelberg New York Tokyo, pp 355–369 (NATO ASI Series, series G, vol 19)
Nover L (ed) (1984) Heat shock response of eukaryotic cells. Springer, Berlin Heidelberg New York Tokyo
Schlesinger MJ, Ashburner M, Tissieres A (eds) (1982) Heat shock. From bacteria to man. Cold Springer Harbor Laboratory, Cold Spring Harbor, NY
Cash J, Wailes GH, Hopkinson J (1915) The British freshwater Rhizopoda and Heliozoa, vol 3, Rhizopoda, part III. The Ray Society, London
— — — — (1981) Ultrastructure and deposition of silica in rhizopod amoebae. In: Simpson TL, Volcani BE (eds) Silicon and siliceous structures in biological systems. Springer, New York Berlin Heidelberg, pp 281–294
Leadbeater BSC (1981) Ultrastructure and deposition of silica in loricate choanoflagellates. In: Simpson TL, Volcani BE (eds) Silicon and siliceous structures in biological systems. Springer, New York Berlin Heidelberg, pp 295–322
—, Hedley RH (1980) An atlas of freshwater testate amoebae. British Museum (Natural History) and Oxford University Press, London and Oxford
Page F (1976) An illustrated key to freshwater and soil amoebae. Freshwater Biological Association, Ambleside
Penard E (1902) Faune rhizopodique du Bassin du Léman. Henry Kündig, Génève
Raikov IB (1982) The protozoan nucleus. Morphology and evolution. Springer, Wien New York [Alfert M et al (eds) Cell biology monographs, vol 9]
Volcani BE (1981) Cell wall formation in diatoms: morphogenesis and biochemistry. In: Simpson TL, Volcani BE (eds) Silicon and siliceous structures in biological systems. Springer, New York Berlin Heidelberg, pp 157–200
— (1983) Aspects of silicification in biological systems. In: Westbroek P, De Jong EW (eds) Biomineralization and biological metal accumulation. Reidel, Dordrecht, pp 389–405
Cleland RE (1987) The mechanism of wall loosening and wall extension. In: Cosgrove DJ, Knievel DP (eds) Physiology of cell expansion during plant growth. American Society of Plant Physiologists, Rockville, MD, pp 18–27
Erickson RO (1980) Microfibrillar structure of growing plant cell wall. In: Getz WM (ed) Mathematical modelling in biology and ecology. Springer, Berlin Heidelberg New York, pp 192–212 (Lecture notes in biomathematics 33)
Levy S (1986) The control of microfibril orientation in the cell wall ofNitella. PhD Thesis, University of Bristol, Bristol, UK
Neville AC (1980) Optical methods in cuticle research. In: Miller TA (ed) Cuticle techniques in arthropods. Springer, Berlin Heidelberg New York, pp 45–89
— — (1985) The helicoidal concept in plant cell wall ultrastructure and morphogenesis. In: Brett CT, Hillman JR (eds) Biochemistry of plant cell walls. Cambridge University Press, Cambridge, pp 99–124 (Society of experimental biology seminars 28)
Richmond PA (1977) Control of plant cell morphogenesis by the cell wall: analysis inNitella. PhD thesis, University of Pennsylvania, Philadelphia, USA
—, Brandes H (1965)Funaria hygrometrica (Musci)-Protonema-Entwicklung. IWF, Göttingen (Encycl Cinematog 962)
Gunning BES (1982) The cytokinetic apparatus. Its development and spatial regulation. In: Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 229–292
Gibbs M (1966) Carbohydrates: their role in plant metabolism and nutrition. In: Steward FC (ed) Plant physiology. Academic Press, New York, pp 3–115
Jensen WA (1962) Botanical histochemistry. WH Freeman, San Francisco
Preiss J, Levi C (1980) Starch biosynthesis and breakdown. In: Preiss J (ed) The biochemistry of plants, carbohydrates: structure and function, vol 3. Academic Press, New York, pp 371–423
Thomson DJ, Bradbury S (1987) An introduction to photomicrography. Oxford Scientific Publications, Oxford (Royal microscopical society handbooks, vol 13)
Van Noorden S (1986) Tissue preparation and immunostaining techniques for light microscopy. In: Polak JM, Van Noorden S (eds) Immunocytochemistry: modern methods and applications, 2nd edn. Wright, Bristol, pp 27–53
Fahn A, Rachmilevitz T (1979) Ultrastructure and nectar secretion inLonicera japonica. In: Fahn A (ed) Secretory tissues in plants. Academic Press, New York, pp 51–65
Fleurat-Lessard P (1990) Structure and ultrastructure of the pulvinus in nyctinastic legumes. In: Satter RL et al (eds) The pulvinus: motor organ of leaf movement. American Society of Plant Physiologists, Rockville, MD, pp 101–129
Fromm J, Eschrich W (1990) Scismonastic movements inMimosa. In: Satter RL et al (eds) The pulvinus: motor organ of leaf movement. American Society of Plant Physiologists, Rockville, MD, pp 25–43
Holloway PJ (1981) Structure and histochemistry of plant cuticular membranes: an overview. In: Cutler DF, Alvin KL, Price CE (eds) The plant cuticle. Academic Press, New York, pp 1–32
Lee Y (1990) Ion movements that control pulvinar curvature in nyctinastic legumes. In: Satter RL et al (eds) The pulvinus: motor organ of leaf movement. American Society of Plant Physiologists, Rockville, MD, pp 130–141
Pfeffer W (1906) The physiology of plants, vol 3. Oxford University Press, Oxford
Satter RL (1990) Leaf movement: an overview of the field. In: Satter RL et al (eds) The pulvinus: motor organ of leaf movement. American Society of Plant Physiologists, Rockville, MD, pp 1–9
Satter RL, Gorton HL, Vogelmann TC (eds) (1990) The pulvinus: motor organ of leaf movement. American Society of Plant Physiologists, Rockville, MD (Current topics in plant physiology, vol 3)
Vincent JF (1982) Structural biomaterials. Halsted Press, New York
York WS, Darvill AG, McNeil M, Stevenson TT, Albersheim P (1986) Isolation and characterization of plant cell walls and cell wall components. In: Weissbach A, Weissbach H (eds) Methods in enzymology, vol 118. Academic Press, New York, pp 3–40
Allan EF, Trewavas AJ (1987) The role of calcium in metabolic control. In: Davies D (ed) The biochemistry of plants, vol 12. Academic Press, New York, pp 117–149
Olive LS (1975) The mycetozoans. Academic Press, New York
— (1982) Eumycetozoa. In: Parker SP (ed) Synopsis and classification of living organisms. McGraw-Hill, New York, pp 521–525
Roos UP, Guhl B (1990) Microtubules in interphase and mitosis of cellular slime molds. In: Akkas N (ed) Biomechanics of active movement and deformation of cells. Springer, Berlin Heidelberg New York Tokyo, pp 73–107 (NATO ASI Series, series H, vol 42)
— (1990) Phylum plasmodial slime molds, class protostelids. In: Margulis L, Corliss JO, Melkonian M, Chapman DJ (eds) Handbook of protoctista. Jones and Bartlett, Boston, pp 484–497
Bewley JD, Black M (1978) Physiology and biochemistry of seeds in relation to germination. Springer, Berlin Heidelberg New York
Bullock S, Willetts HJ, Ashford AE (1980 a) The structure and histochemistry of sclerotia ofSclerotinia minor Jagger I. Light and electron microscope studies on sclerotial development. Protoplasma 104: 315–331
—, Ashford AE, Willetts HJ (1980 b) The structure and histochemistry of sclerotia ofSclerotinia minor Jagger II. Histochemistry of extracellular substances and cytoplasmic reserves. Protoplasma 104: 333–351
—, Willetts HJ, Ashford AE (1983) The structure and histochemistry of sclerotia ofSclerotinia minor Jagger III. Changes in ultrastructure and loss of reserve materials during carpogenic germination. Protoplasma 117: 214–225
Coley-Smith JR, Cooke RC (1971) Survival and germination of fungal sclerotia. Annu Rev Phytopathol 9: 65–92
Ebel JP, Colas J, Muller S (1958) Recherches cytochimiques sur les polyphosphates inorganiques contenus dans les organismes vivants II. Mise au point de méthodes de détection cytochimiques spécifiques des polyphosphates. Exp Cell Res 15: 28–36
Feder N, O'Brien TP (1968) Plant microtechnique: some principles and new methods. Amer J Bot 55: 123–142
Fisher DB (1968) Protein staining of ribboned epon sections for light microscopy. Histochemie 16: 92–96
Fox FM (1986 a) Ultrastructure and infectivity of sclerotium-like bodies of the ectomycorrhizal fungusHebeloma sacchariolens, on birch (Betula spp.). Trans Br Mycol Soc 87: 359–369
— (1986 b) Ultrastructure and infectivity of sclerotia of the ectomycorrhizal fungusPaxillus involutus on birch (Betula spp.). Trans Br Mycol Soc 87: 627–658
Grenville DJ, Peterson RL, Piché Y (1985 a) The development, structure, and histochemistry of sclerotia of ectomycorrhizal fungi. II.Paxillus involutus. Can J Bot 63: 1412–1417
—, Piché Y, Peterson RL (1985 b) Sclerotia as viable sources of mycelia for the establishment of ectomycorrhizae. Can J Microbiol 31: 1085–1088
Insell JP, Huner NPA, Newsted WJ, van Huystee RB (1985) Light microscopic and polypeptide analyses of sclerotia from mesophilic and psychrophilic pathogenic fungi. Can J Bot 63: 2305–2310
Kohn LM, Grenville DJ (1989 a) Anatomy and histochemistry of stromatal anamorphs in the Sclerotiniaceae. Can J Bot 67: 371–393
— — (1989 b) Ultrastructure of stromatal anamorphs in the Sclerotiniaceae. Can J Bot 67: 394–406
Laiho O (1970)Paxillus involutus us a mycorrhizal symbiont of forest trees. Acta For Fenn 106: 1–72
Ling-Lee M, Chilvers GA, Ashford AE (1975) Polyphosphate granules in three different kinds of tree mycorrhiza. New Phytol 75: 551–554
— — — (1977) A histochemical study of phenolic materials in mycorrhizal and uninfected roots ofEucalyptus fastigata Deane and Maiden. New Phytol 78: 313–328
Lott JNA (1981) Protein bodies in seeds. Nord J Bot 1: 421–432
—, Spitzer E (1980) X-ray analysis studies of elements stored in protein body globoid crystals ofTriticum grains. Plant Physiol 66: 494–499
Marx DH, Bryan WC (1975) Growth and ectomycorrhizal development of loblolly pine seedlings in fumigated soil infested with the fungal symbiontPisolithus tinctorius. Forest Sci 21: 245–254
Massaro EJ, Markert CL (1968) Protein staining on starch gels. J Histochem Cytochem 16: 380–382
Massicotte HB, Ackerley CA, Peterson RL (1985) An improved fixation protocol for ultrastructural studies of ectomycorrhizae. Proc Microsc Soc Can 12: 68–69
Newsted WJ, Huner NPA (1988) Major sclerotial polypeptides of psychrophilic pathogenic fungi: intracellular localization and antigenic relatedness. Protoplasma 147: 162–169
Novak LA, Kohn LM (1988) Electrophoresis of major proteins in stromata of members of the Sclerotiniaceae. Trans Br Mycol Soc 91: 639–647
O'Brien TP, McCully ME (1981) The study of plant structure: principles and selected methods. Termarcarphi, Sydney
Orlovich DA, Ashford AE, Cox GC (1989) A reassessment of polyphosphate granule composition in the ectomycorrhizal fungusPisolithus tinctorius. Aust J Plant Physiol 16: 107–115
Russo GM, van Etten JL (1985) Synthesis and localization of a development-specific protein in sclerotia ofSclerotinia sclerotiorum. J Bacteriol 163: 696–703
Saito I (1974) Ultrastructural aspects of the maturation of sclerotia ofSclerotinia sclerotiorum (Lib.) de Bary. Trans Mycol Soc Jap 15: 384–400
Sauter JJ, Wellenkamp S (1988) Protein storing vacuoles in ray cells of willow wood (Salix caprea L.). IAWA Bull 9: 59–65
Willetts HJ (1971) The survival of fungal sclerotia under adverse environmental conditions. Biol Rev 46: 387–407
—, Bullock S, Begg E, Matsumoto N (1990) The structure and histochemistry of sclerotia ofTyphula incarnata Lasch: Fr. Can J Bot 68: 2083–2091
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moore, A.E.P., Ashford, A.E. & Peterson, R.L. Reserve substances inPaxillus involutus sclerotia. Protoplasma 163, 67–81 (1991). https://doi.org/10.1007/BF01323331
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01323331