Summary
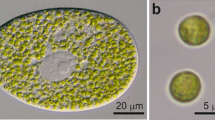
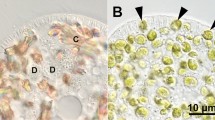
After treatment with the carboxylic ionophore monensin theChlorella containing perialgal vacuoles of the “green”Paramecium bursaria swell. TheParamecium cells remain motile at this concentration for at least one day. The swelling is only observed in illuminated cells and can be inhibited by DCMU. We assume that during photosynthesis the perialgal vacuoles are acidified and that monensin exchanges H+ ions against monovalent cations (here K+). In consequence the osmotic value of the vacuoles increases. The proton gradient is believed to drive the transport of maltose from the symbiont into the host. Another but light independent effect of the monensin treatment is the swelling of peripheral alveoles of the ciliates, likewise indicating that the alveolar membrane contains an active proton pump.
Similar content being viewed by others
Abbreviations
- HEPES:
-
N-(2-hydroxyethyl)piperazine-N′-2-ethane sulfonic acid
- DCMU:
-
3-(3, 4-dichlorophenyl)-1,1-dimethylurea
References
Boss WF (1984) Monensin-induced swelling of Golgi apparatus cisternae mediated by a proton gradient. Eur J Cell Biol 34: 1–8
Cernichiari E, Muscatine L, Smith DC (1969) Maltose excretion by the symbiotic algae ofHydra viridis. Proc R Soc Lond [Biol] 173: 557–576
Fischer A, Meindl D, Loos E (1989) Glucose excretion by the symbioticChlorella ofSpongilla fluviatilis. Planta 179: 251–256
Geisow MJ, Burgoyne RD (1982) Effect of monensin on chromaffin cells and the mechanism of organelle swelling. Cell Biol Int Rep 6: 933–939
Hohman TC, McNeil PL, Muscatine L (1982) Phagosome-lysome fusion inhibited by algal symbionts ofHydra viridis. J Cell Biol 94: 56–63
Kessler E, Kauer G, Rahat M (1991) Excretion of sugars byChlorella species capable and incapable of symbiosis withHydra viridis. Bot Acta 104: 58–63
Kleinig H, Sitte P (1986) Zellbiologie, 2nd edn. Fischer, Stuttgart
Larsen J, Satir P (1991) Analysis of Ni2+-induced arrest ofParamecium axonemes. J Cell Sci 99: 33–40
Matzke B, Schwarzmeier E, Loos E (1990) Maltose excretion by the symbioticChlorella of the helizoanAcanthocystis turfacae. Planta 181: 593–598
Mollenhauer HH, Morré DJ, Droleskey R (1983) Monensin affects the trans half ofEuglena dictyosomes. Protoplasma 114: 119–124
Muscatine L, Karakashian SJ, Karakashian MW (1967) Soluble extracellular products of algae symbiotic with a ciliate, a sponge and a mutantHydra. Comp Biochem Physiol 20: 1–12
Pressmann BC (1976) Biological applications of ionophores. Annu Rev Biochem 45: 501–528
Reisser W (1976) Die stoffwechselphysiologischen Beziehungen zwischenParamecium bursaria Ehrbg. undChlorella spec., in derParamecium bursaria-Symbiose. II. Symbiose-spezifische Merkmale der Stoffwechselphysiologie und der Cytologie des Symbioseverbandes und ihre Regulation. Arch Microbiol 111: 161–170
— (1981) Theendosymbiontic unit ofStentor polymorphus andChlorella sp. Morphological and physiological studies. Protoplasma 105: 273–284
— (1986) Endosymbiotic associations of freshwater protozoa and algae. Prog Protistol 1: 195–214
— (1990) Participation of algal cell wall surface structures in the formation of the host-symbiont-interface of endocytobiotic systems. Exp Phycol 1: 55–68
Sandeaux R, Sandeaux J, Gavach C, Bernhard B (1982) Transport of Na+ by monensin across biomolecular lipid membranes. Biochim Biophys Acta 684: 127–132
Smith DC, Douglas AE (1987) The biology of symbiosis. E Arnold, London
Stelly N, Mauger J-P, Claret M, Adoutte A (1991) Cortical alveoli ofParamecium: a vast submembraneous calcium storage compartment. J Cell Biol 113: 103–112
Tartakoff AM (1983) Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell 32: 1026–1028
—, Vassalli P (1981) Plasma cell endocytosis: is it related to immunoglobulin secretion? Eur J Cell Biol 26: 188–197
Willenbrink J (1987) Die pflanzliche Vacuole als Speicher. Naturwissenschaften 74: 22–29
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schüßler, A., Schnepf, E. Photosynthesis dependent acidification of perialgal vacuoles in theParamedum bursaria/Chlorella symbiosis: Visualization by monensin. Protoplasma 166, 218–222 (1992). https://doi.org/10.1007/BF01322784
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01322784