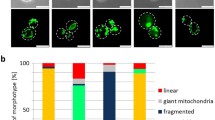
Abstract
The mitochondrial genome of animals encodes a few subcomponents of the respiratory chain complexes I, III and IV, whereas nuclear DNA encodes the overwhelming majority, both in quantitative and qualitative terms, of mitochondrial proteins. Complete depletion of mitochondrial DNA (mtDNA) can be achieved by culturing cells in the presence of inhibitors of mtDNA replication or mitochondrial protein synthesis, giving rise to mutant cells (ϱ∘ cells) which carry morphological near-to-intact mitochondria with respiratory defects. Such cells can be used to study the impact of mitochondrial respiration on apoptosis. ϱ∘ cells do not undergo cell death in response to determined stimuli, yet they conserve their potential to undergo full-blown apoptosis in many experimental systems. This indicates that mtDNA and associated functions (in particular mitochondrial respiration) are irrelevant to apoptosis execution. However, the finding that mtDNA-deficient mitochondria can undergo apoptosis does not argue against the involvement of mitochondria in the apoptotic process, since mitochondria from ϱ∘ cells conserve most of their functions including those involved in the execution of the death programme: permeability transition and release of one or several intermembrane proteins causing nuclear apoptosis.
Similar content being viewed by others
References
Barr PJ, Tomei LD. Apoptosis and its role in human disease.Biotechnol 1994;12: 487–493.
Thompson CB. Apoptosis in the pathogenesis and treatment of disease.Science 1995;267: 1456–1462.
Kroemer G, Petit PX, Zamzami N, Vayssière J-L, Mignotte B. The biochemistry of apoptosis.FASEB J 1995;9: 1277–1287.
Kroemer G. The pharmacology of T cell apoptosis.Adv Immunol 1995;58: 211–296.
Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis.Immunol Today 1996; in press.
Ishizaki Y, Cheng L, Mudge AW, Raff MC. Programmed cell death by default in embryonic cells, fibroblasts, and cancer cells.Mol Biol Cell 1995;1995: 1443–1458.
Weil M, Jacobson MD, Coles HSR,et al. Constitutive expression of the machinery for programme cell death.J Cell Biol 1996;133: 1053–1059.
Jacobson MD, Burne JF, Raff MC. Programmed cell death and Bcl-2 protection in the absence of a nucleus.EMBO J 1994;13: 1899–1910.
Schulze-Osthoff K, Walczak H, Droge W, Krammer PH. Cell nucleus and DNA fragmentation are not required for apoptosis.J Cell Biol 1994;127: 15–20.
Nakajima H, Golstein P, Henkart PA. The target cell nucleus is not required for cell-mediated granzyme- or Fas-based cytotoxicity.J Exp Med 1995;181: 1905–1909.
Borst P, Grivell LA, Groot GSP. Organelle DNA.Trends Biochem Sci 1984;9: 128–130.
Gray MW. Origin and evolution of mitochondrial DNA.Annu Rev Biochem 1989;5: 25–50.
Schatz G, Dobberstein B. Common principles of protein translocation across membranes.Science 1996;271: 1519–1526.
King MP, Attardi G. Isolation of human cell lines lacking mitochondrial DNA.Meth Enzymol 1996;264: 304–313.
Baixeras E, Bosca L, Stauber C,et al. From apoptosis to autoimmunity: Insights from the signaling pathways leading to proliferation or to programmed cell death.Immunol Rev 1994;142: 53–91.
Skowronek P, Haferkamp O, Rödel G. A fluorescence-microscopic and flow-cytometric study of HELA cells with an experimentally induced respiratory deficiency.Biochem Biophys Res Comm 1992;187: 991–998.
Marchetti P, Susin SA, Decaudin D,et al. Apoptosis-associated derangement of mitochondrial function in cells lacking mitochondrial DNA.Cancer Res 1996;56: 2033–2038.
King M, Attardi G. Mityochondria-mediated transformation of human rho∘ cells.Meth Enzymol 1996;264: 313–320.
Schulze-Osthoff K, Bakker AC, Vanhaesbroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions.J Biol Chem 1992;267: 5317–5323.
Schulze-Osthoff K, Beyaert R, Vandevoorde V, Haegeman G, Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF.EMBO J 1993;12: 3095–3104.
Hennet T, Richter C, Peterhans E. Tumour necrosis factoralpha induces Superoxide anion production in mitochondria of L929 cells.Biochem J 1993;289: 587–592.
Schulze-Osthoff K, Krammer PH, Dröge W. Divergent signalling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death.EMBO J 1994;13: 4587–4596.
Jacobson MD, Burne JF, King MP, Miyashita T, Reed JC, Raff MC. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA.Nature 1993;361: 365–369.
Gamen S, Anel A, Montoya J, Marzo I, Piñeiro A, Naval J. mtDNA-depleted U937 cells are sensitive to TNF and Fasmediated cytotoxicity.FEBS Lett 1995;376: 15–18.
Wolvetang EJ, Johnson KL, Krauer K, Ralph SJ, Linnane AW. Mitochondrial respiratory chain inhibitors induce apoptosis.FEBS Lett 1994;339: 40–44.
Shimizu S, Eguchi Y, Kosaka H, Kamlike W, Matsuda H, Tsujimoto Y. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL.Nature 1995;374: 811–813.
Yoneda M, Kasumata K, Hayakawa M, Tanaka M, Ozawa T Oxygen stress induces an apoptotic cell death associated with fragmentation of mitochondrial genome.Biochem Biophys Res Comm 1995;209: 723–729.
McLauglin KA, Osborne BA, Goldsby RA. The role of oxygen in thymocyte apoptosis.Eur J Immunol 1996;26: 1170–1174.
Jacobson MD, Raff MC. Programmed cell death and Bcl-2 protection in very low oxygen.Nature 1995;374: 814–816.
Hug H, Enari M, Nagata S. No requirement of reactive oxygen intermediates in Fas-mediated apoptosis.FEBS Lett 1994;351: 311–313.
Greenhalf W, Stephan C, Chaudhuri B. Role of mitochondria and C-terminal membrane anchor of Bcl-2 in Bax induced growth arrest and mortality inSacharomyces cerevisiae. FEBS Lett 1996;380: 169–175.
Tepper CG, Studzinski GP. Teniposide induces nuclear but not mitochondrial DNA degradation.Cancer Res 1992;52: 3384–3390.
Tepper CG, Studzinski GP. Resistance of mitochondrial DNA to degradation characterizes the apoptotic but not the necrotic mode of human leukemia cell death.J Cell Biochem 1993;52: 352–361.
Vayssière J-L, Petit PX, Risler Y, Mignotte B. Commitment to apoptosis is associated with changes in mitochondrial biogenesis and activity in cell lines conditionally immortalized with simian virus 40.Proc Natl Acad Sci USA 1994;91: 11752–11756.
Zamzami N, Marchetti P, Castedo M,et al. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte deathin vivo. J Exp Med 1995;181: 1661–1672.
Petit PX, LeCoeur H, Zorn E, Dauguet C, Mignotte B, Gougeon ML. Alterations of mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis.J Cell Biol 1995;130: 157–167.
Zamzami N, Marchetti P, Castedo M,et al. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death.J Exp Med 1995;182: 367–377.
Castedo M, Macho A, Zamzami N,et al. Mitochondrial perturbations define lymphocytes undergoing apoptotic depletionin vivo. EurJ Immunol 1995;25: 3277–3284.
Castedo M, Hirsch T, Susin SA,et al. Sequential acquisition of mitochondrial and plasma membrane alterations during early lymphocyte apoptosis.J Immunol 1996;157: 512–521.
Marchetti P, Castedo M, Susin SA,et al. Mitochondrial permeability transition is a central coordinating event of apoptosis.J Exp Med 1996; in press, Sept.
Macho A, Decaudin D, Castedo M,et al. Chloromethyl-Xrosamine — An aldehyde-fixable potential-sensitive fluorochrome for the detection of early apoptosis.Cytometry 1996; in press.
Attardi G, Schatz G. Biogenesis of mitochondria.Ann Rev Cell Biol 1988;4: 289–333.
Osborne BA, Smith SW, Liu Z-G, McLaughlin KA, Grimm L, Schwanz LM. Identification of genes induced during apoptosis in T cells.Immunol Rev 1994;142: 301–320.
Zoratti M, Szabò I. The mitochondrial permeability transition.Biochem Biophys Acta — Rev Biomembr 1995;1241: 139–176.
Bernardi P, Petronilli V. The permeability transition pore as a mitochondrial calcium release channel; a critical appraisal.J Bioenerg Biomembr 1996;28: 129–136.
Zamzami N, Marchetti P, Castedo M,et al. Inhibitors of permeability transition interfere with the disruption of the mitochondrial transmembrane potential during apoptosis.FEBS Lett 1996;384: 53–57.
Marchetti P, Hirsch T, Zamzami N,et al. Mitochondrial permeability transition triggers lymphocyte apoptosis.J Immunol 1996; in press.
Zamzami N, Susin SA, Marchetti P,et al. Mitochondrial control of nuclear apoptosis.J Exp Med 1996;183: 1533–1544.
Susin S, Zamzami N, Castedo M,et al. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease.J Exp Med 1996;184: 1331–1342.
Martin SJ, Newmeyer DD, Mathisa S,et al. Cell-free reconstitution of Fas-, UV radiation- and ceramide-induced apoptosis.EMBO J 1995;14: 5191–5200.
Liu X, Kim CN, Yang I, Jemmerson R, Wang X. Induction of apoptic program in cell-free extracts: requirement for dATP and cytochrome C.Cell 1996;86: 147–157.
Fearnhead HO, Dinsdale D, Cohen GM. An interleukin-1 beta-converting enzyme-like protease is a common mediator of apoptosis in thymocytes.FEBS Lett 1995;375: 283–288.
Zhu HJ, Fearnhead HO, Cohen GM. An ICE-like protease is a common mediator of apoptosis induced by diverse stimuli in human monocytic THP.1 cells.FEBS Lett 1995;374: 303–308.
Cain K, Inayathussain SH, Couet C, Cohen GM. A cleavage-site-directed inhibitor of interleukin 1 beta-converting enzyme-like proteases inhibits apoptosis in primary cultures of rat hepatocytes.Biochem J 1996;314: 27–32.
Slee EA, Zhu HJ, Chow SC, Macfarlane M, Nicholson DW, Cohen GM. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.fmk) inhibits apoptosis by blocking the processing of CPP32 Biochem J 1996;315 21–24.
Pronk GJ, Ramer K,Amiri P, Williams LT. Requirement of an ICE-like protease for induction of apoptosis and ceramide generation by REAPER.Science 1996;271: 808–810.
Jacobson MD, Weil M, Raff MC. Role of Ced-3IICE-family proteases in staurosporine-induced programmed cell death.J Cell Biol 1996;133: 1041–1051.
Bernardi P, Broekemeier KM, Pfeiffer DR. Recent progress on regulation of the mitochondrial permeability transition pore; a cyclosporin-sensitive pore in the inner mitochondrial membrane.J Bioenerg Biomembr 1994;26: 509–517.
Brandolin G, Le-Saux A, Trezeguet V, Lauquinn GJ, Vignais PV.Chemical, immunological, enzymatic, and genetic approaches to studying the arrangement of the peptide chain of the ADPIATP carrier in the mitochondrial membrane.J Bioenerg Biomembr 1993;25: 493–501.
Gamen S, Marzo S, Anel A, Piñeiro A, Naval J. CPP32 inhibition prevents Fas-induced ceramide generation and apoptosis in human cells.FEBS Lett 1996;390: 233–237.
Newmeyer DD, Farschon DM, Reed JC. Cell-free apoptosis in xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria.Cell 1994;79: 353–364.
Author information
Authors and Affiliations
Additional information
Supported by ARC, ANRS, CNRS, FRM, Fondation de France, INSERM, NATO, Ligue contre le Cancer Ministère de la Recherche et de l'Industrie (France), and Sidaction (to GK). SAS receives a fellowship from the Spanish Government (Ministerio de Ciencia y Educación).
Rights and permissions
About this article
Cite this article
Marchetti, P., Zamzami, N., Susin, S.A. et al. Apoptosis of cells lacking mitochondrial DNA. Apoptosis 1, 119–125 (1996). https://doi.org/10.1007/BF01321017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01321017