Abstract
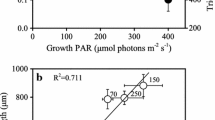
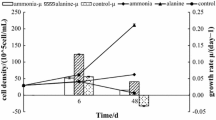
Toxin content (fmol cell−1) and a suite of elemental and macromolecular variables were measured in batch cultures of the dinoflagellatesAlexandrium fundyense, A. tamarense andAlexandrium sp. from the southern New England region, USA. A different perspective was provided by semicontinuous cultures which revealed sustained, steady-state physiological adaptations by cells to N and P limitation. Two types of variability were investigated. In batch culture, changes in nutrient availability with time caused growth stage variability in toxin content, which often peaked in mid-exponential growth. A second type of variability that could be superimposed on growth stage differences is best exemplified by the high toxin content of cells grown at suboptimal temperatures. Calculations of the net rate of toxin production (R tox ; fmol cell−1 d−1) for these different culture treatments and modes made it possible to separate the dynamics of toxin production from cell division. Over a wide range of growth rates, cells produced toxin at rates approximating those needed to replace “losses” to daughter cells during division. The exception to this direct proportionality was with P limitation, which was associated with a dramatic increase in the rate of toxin production as cells stopped dividing due to nutrient limitation in batch culture. Growth stage variability in batch culture thus reflects small imbalances (generally within a factor of two) between the specific rates of toxin production and cell division. N limitation and CO2 depletion both affect pathways involved in toxin synthesis before those needed for cell division; P limitation does the opposite. The patterns of toxin accumulation were the same as for major cellular metabolites or elemental pools. The highest rates of toxin production appear to result from an increased availability of arginine (Arg) within the cell, due to either a lack of competition for this amino acid from pathways involved in cell division or to increased de novo synthesis. There were no significant changes in toxin content with either acclimated growth at elevated salinity, or with short term increases or decreases of salinity. These results demonstrate that toxin production is a complex process which, under some conditions, is closely coupled to growth rate; under other conditions, these processes are completely uncoupled. Explanations for the observed variability probably relate to pool sizes of important metabolites and to the differential response of key biochemical reactions to these pool sizes and to environmental conditions.
Similar content being viewed by others
Literature cited
Achituv, M., Bar-Akiva, A. (1973). Nitrogen accumulation induced by phosphorus deficiency in citrus plants. Sci. Hortic. 1: 251–252
Alam, M. I., Hsu, C. P., Shimizu, Y. (1979). Comparison of toxins in three isolates ofGonyaulax tamarensis (Dinophyceae). J. Phycol. 15: 106–110
Anderson, D. M., Lindquist, N. L. (1985). Time-course measurements of phosphorus depletion and cyst formation in the dinoflagellateGonyaulax tamarensis Lebour. J. exp. mar. Biol. Ecol. 86: 1–13
Anderson, D. M., Kuhs, D. M., Sullivan, J. J., Hall S. S. (In press). Toxin composition variations in one isolate of the dinoflagellateAlexandrium fundyense. Toxicon
Balech, E. (1985). The genusAlexandrium orGonyaulax of the tamarensis group. In: Anderson, D. M., White, A. W., Baden, D. G. (eds.) Toxic dinoflagellates. Elsevier, New York, p. 33–38
Binder, B. J. (1986). The physiology of dormancy and germination in cysts of the marine dinoflagellateScrippsiella trochoidea. Ph. D. Thesis, Massachusetts Institute of Technology, Massachusetts
Boczar, B. A., Beitler, M. A., Liston, J., Sullivan, J. J., Cattolico, R. A. (1988). Paralytic shellfish toxins inProtogonyaulax tamarensis andProtogonyaulax catenella in axenic culture. Pl. Physiol., Wash. 88: 1285–1290
Boyer, G. L., Sullivan, J. J., Andersen, R. J., Harrison, P. J., Taylor, F. J. R. (1987). Effects of nutrient limitation on toxin production and composition in the marine dinoflagellateProtogonyaulax tamarensis. Mar. Biol. 96: 123–128
Bradford, M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 72: 248–254
Brand, L. E., Guillard, R. R. L., Murphy, L. S. (1981). A method for the rapid and precise determination of acclimated phytoplankton reproduction rates. J. Plankton Res. 3: 193–201
Cembella, A. E., Sullivan, J. J., Boyer, G. L., Taylor, F. J. R., Anderson, R. J. (1987). Variation is paralytic shellfish toxin composition within theProtogonyaulax tamarensis/catenella species complex: red tide dinoflagellates. Biochem. Syst. Ecol. 15: 171–186
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analyt. Chem. 28: 350–356
Hall, S. (1982). Toxins and toxicity ofProtogonyaulax from the northeast Pacific. Ph.D. Thesis, University of Alaska
Heinbokel, J. F. (1978). Studies on the functional role of tintinnids in the Southern California Bight. I. Grazing and growth rates in laboratory cultures. Mar. Biol. 47: 177–189
Keller, M. D., Selvin, R. C., Claus, W., Guillard, R. R. L. (1987). Media for the culture of oceanic ultraphytoplankton. J. Phycol. 23: 633–638
Kodama, M., Ogata, T. (1988). New insights into shellfish toxins. Mar. Pollut. Bull. 19: 559–564
Li, W. K. W., Glover, H. E., Morris, I. (1980). Physiology of carbon photoassimilation byOscillatoria thiebautii in the Caribbean Sea. Limnol. Oceanogr. 25: 447–456
Maranda, L., Anderson, D. M., Shimizu, Y. (1985). Comparison of toxicity between populations ofGonyaulax tamarensis of eastern North American waters. Estuar. cstl Shelf Sci. 21: 401–410
Mopper, K., Lindroth, P. (1982). Diel and depth variations in dissolved free amino acids and ammonium in the Baltic Sea determined by shipboard HPLC analysis. Limnol. Oceanogr. 27: 336–347
Ogata, T., Ishimaru, T., Kodama, M. (1987). Effect of water temperature and light intensity on growth rate and toxicity change inProtogonyaulax tamarensis. Mar. Biol. 95: 217–220
Oshima, Y., Yasumoto, T. (1979). Analysis of toxins in culturedGonyaulax excavata cells originating in Ofunato Bay, Japan. In: Taylor, D. L., Seliger, H. E. (eds.) Toxic dinoflagellate blooms. Elsevier, New York, p. 377–380
Prakash, A. (1967). Growth and toxicity of a marine dinoflagellate. J. Fish. Res. Bd Can. 24: 1589–1606
Proctor, N. H., Chan, S. L., Trevor, A. J. (1975). Production of saxitoxin by cultures ofGonyaulax catenella. Toxicon 13: 1–9
Rabe, E., Lovatt, C. J. (1986). Increased arginine biosynthesis during phosphorus deficiency. Pl. Physiol., Wash. 181: 774–779
Schmidt, R. J., Loeblich, A. R. III (1979). A discussion of the systematics of toxicGonyaulax species containing paralytic shellfish poison. In: Taylor, D. L., Seliger, H. H. (eds.) Toxic dinoflagellate blooms. Elsevier, New York, p. 83–88
Shimizu, Y. (1979). Developments in the study of paralytic shellfish toxins. In: Taylor, D. L., Seliger, H. H. (eds.) Toxic dinoflagellate blooms. Elsevier, New York, p. 321–326
Shimizu, Y. (1987). Dinoflagellate toxins. In: Taylor, F. J. R. (ed.) The biology of dinoflagellates. Blackwell Scientific, Oxford, p. 282–315
Shimizu, Y., Norte, M. A., Hori, A., Genenah A., Kobayashi, M. (1984). Biosynthesis of saxitoxin analogues: the unexpected pathway. J. Am. chem. Soc. 106: 6433–6434
Spector, T. (1978). Refinement of the Coomassie blue method of protein quantitation. Analyt. Biochem. 86: 142–146
Strickland, J. D. H., Parsons, T. R. (1972). A practical handbook of seawater analysis. Fish. Res. Bd Can. Bull No. 167
Sullivan, J. J. (in press). Applications of an HPLC method in PSP research. In: Hall, S., Strichartz, G. (eds.) Marine toxins: origins, structure and molecular pharmacology. Am. chem. Soc. Symp. Ser., Am. chem. Soc., Washington, D.C.
Sullivan, J. J., Wekell, M. M. (1988). The application of HPLC in a PSP monitoring program. In: Kramer, E. E., Liston, J. (eds.) Seafood quality determination. Sea Grant College Program, University of Alaska, Anchorage, p. 357–371
White, A. W. (1978). Salinity effects on growth and toxin content ofGonyaulax excavata, a marine dinoflagellate causing paralytic shellfish poisoning. J. Phycol. 14: 475–479
White, A. W., Maranda, L. (1978). Paralytic toxins in the dinoflagellateGonyaulax excavata and in shellfish. J. Fish. Res. Bd Can. 35: 397–402
Author information
Authors and Affiliations
Additional information
Communicated by J. Grassle, Woods Hole
Rights and permissions
About this article
Cite this article
Anderson, D.M., Kulis, D.M., Sullivan, J.J. et al. Dynamics and physiology of saxitoxin production by the dinoflagellatesAlexandrium spp.. Mar. Biol. 104, 511–524 (1990). https://doi.org/10.1007/BF01314358
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01314358