Abstract
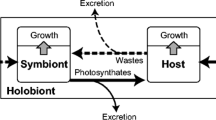
Factors were examined that affect survival and growth of two common species of large foraminifera from the Red Sea,Amphisorus hemprichii Ehrenberg andAmphistegina lobifera Larsen, 1976. The former is host for dinoflagellate and the latter for diatom zooxanthellae. Experimental conditions were modeled on conditions at 25 m during spring at Wadi Taba, Gulf of Elat, Israel, the season and site where the experimental organisms were collected between 1983 and 1988. The two species responded quite differently in nutritional experiments.A. hemprichii grew, on average, 0.270 mm in diameter in 3 mo on a diet ofNitzschia subcommunis Hustedt,Chlorella sp. (clone AT) orCylindrotheca closterium Rabenhorst isolated from their native habitat. Unfed controls did not grow. In contrast, unfed populations ofA. lobifera grew as well or better than those that were fed unialgal diets. Growth of both species was enhanced on particular mixed algal diets. Both species required photosynthetically active symbionts. Even when fed weekly and supplied with nutrients, neither species survived in the dark. All individuals ofA. hemprichii died after 8 wk incubation in the dark;A. lobifera survived longer, but all were dead by 13 wk. The highest growth rate ofA. hemprichii (0.037 mm wk−1) was obtained when they were fed, the medium was enriched, and the medium was changed weekly. All other conditions being the same, growth rate dropped to 0.009 mm wk−1 when the medium was changed every 3 wk. In contrast,A. lobifera grew fastest when the medium was changed every 3 wk. Food or enrichment with nitrate or phosphate did not stimulate growth (0.03 mm wk−1) over that of the controls. Specimens ofMarginopora kudakajimensis Gudmundsson from Japan, another dinoflagellate-bearing species, were also tested. They grew best (0.02 mm wk−1) when cultured in light, in media enriched with nitrate and phosphate changed weekly, and fed. All three species withdrew nitrate and phosphate from the medium in chemostat experiments.
Similar content being viewed by others
Literature cited
Arnold, Z. M. (1974). Field and laboratory techniques for the study of living foraminifera. In: Hedley, R. H., Adams, C. G. (eds.)Foraminifera, Vol. 1. Academic Press, London, p. 153–206
Chen, P. S. Jr., Toribara, T. Y., Warner, H. (1956). Microdetermination of phosphorus. Analyt. Chem. 32: 1756–1758
Gudmundsson, G. (1989). Systematics of recent species of the superfamily Soritacea Ehrenberg 1839. Ph.D. dissertation, City University of New York. University Microfilms, Ann Arbor
Hallock, P. (1979). Trends in test shape in large, symbiont-bearing foraminifera. J. foraml Res. 9: 61–69
Hallock, P. (1980). The application of ecologic studies of living algal symbiont-bearing foraminifera to paleoeologic interpretation (abstract). Bull. Am. Ass. Petrol. Geol. 47: 716–717
Hallock, P. (1981). Light dependence inAmphistegina. J. foraml Res. 11: 40–46
Hallock, P. (1985). Why are large Foraminifera large? Paleobiology 11: 195–208
Hallock, P., Cottey, T. L., Forward, L. B., Halas, J. (1986). Population biology and sediment production ofArchaias angulatus (Foraminiferida) in Largo Sound, Florida. J. foraml Res. 16: 1–18
Hottinger, L. (1977). Distribution of larger Peneroplidae,Borelis and Nummulitidae in the Gulf of Elat, Red Sea. Utrecht micropaleont. Bull. 15: 35–109
Jørgensen, B. B., Erez, J., Revsbech, N. P., Cohen, Y. (1985). Symbiotic photosynthesis in a planktonic foraminifera,Globigerinoides sacculifer (Brady) studied with microelectrodes. Limnol. Oceanogr. 30: 1253–1267
Koestler, R. J., Lee, J. J., Reidy, J., Sheryll, R. P., Xenophonotos, X. (1985). Cytological investigation of digestion and re-establishment of symbiosis in the larger benthic foraminiferaAmphistegina lessonii. Endocytobiol. Cell Res. 2: 21–54
Kuile, B. ter, Erez, J. (1984).In situ growth rate experiments on the foraminiferaAmphistegina lobifera andAmphisorus hemprichii. J. foraml Res. 14: 262–276
Kuile, B. ter, Erez, J., Lee, J. J. (1987). The role of feeding in hte metabolism of larger symbiont-bearing foraminifera. Symbioses 4: 335–350
Larsen, A. R. (1976). Studies of recentAmphisteginga, taxonomy and some ecological aspects. Israel J. Earth-Sciences 25: 1–26
Lee, J. J. (1974). Towards understanding the niche of foraminifera. In: Hedley, R. H., Adams, C. G. (eds.) Foraminifera, Vol. 1. Academic Press, London, p. 208–257
Lee, J. J. (1980). Nutrition and physiology of foraminifera. In: Levandowsky, M., Hutner, S. H. (eds.) Biochemistry and physiology of Protozoa, 2nd edn., Vol. 3. Academic Press, New York, p. 43–66
Lee, J. J., Erez, J., Kuile, B. ter., Lagziel, A., Burgos, S. (1988). Feeding rates of two species of larger foraminifera,Amphistegina lobifera andAmphisorus hemprichii, from the Gulf of Elat (Red Sea). Symbioses 5: 61–102
Lee, J. J., McEnery, M. E., Garrison, J. R. (1980a). Experimental studies of larger foraminifera and their symbionts from the Gulf of Elat on the Red Sea. J. foraml Res. 10: 31–47
Lee, J. J., McEnery, M. E., Kennedy, E., Rubin, H. (1975). A nutritional analysis of a sublittoral epiphytic diatom assemblage onEnteromorpha from a Long Island salt marsh. J. Phycol. 21: 14–49
Lee, J. J., McEnery, M. E., Lee, M. J., Reidy, J. J., Garrison, J. R. Röttger, R. (1980b). Algal symbionts in larger foraminifera. In: Schwemmler, N., Schenk, H. E. A. (eds.) Endocytobiology, Vol. 1. Walter de Gruyter & Co., Berlin, p. 113–124
Leutenegger, S. (1984). Symbiosis in benthic foraminifera: specificity and host adaptations. J. foraml Res. 14: 16–35
Levanon-Spanier, I., Padan, E., Reiss, Z. (1979). Primary production in a desert enclosed sea — the Gulf of Elat (Aqaba), Red Sea. Deep-Sea Res. 26: 673–685
Muller, P. H. (1974). Sediment production and population biology of the benthic foraminiferaAmphistegina madagascariensis. Limnol. Oceanogr. 19: 802–809
Muller, P. H. (1977). Some aspects of the ecology of several larger, symbiont-bearing foraminifera and their contribution to warm, shallow water biofacies. Ph.D. dissertation, University of Hawaii, Honolulu (University Microfilms, Doctoral Dissertation Series, No. 77-23491)
Provasoli, L., McLaughlin, J., Droop, M. (1957). The development of artificial media for marine alge. Arch. Mikrobiol. 25: 302–428
Rand, M. C., Greenberg, A. E., Taras, M. J. (eds.) (1976). Standard methods for the examination of water and wastewater, 14th edn. American Public Health Association, Washington, D.C., p. 429–431
Reiss, Z., Hottinger, L. (1984). The Gulf of Aqaba. Ecological micropaleontology. Springer-Verlag, Berlin
Ross, C. A. (1972). Biology and ecology ofMarginopora vertebralis (Foraminifera), Great Barrier Reef. J. Protozool. 19: 181–192
Röttger, R. (1972a). Die Bedeutung der Symbiose vonHeterostegina depressa (Foraminifera, Nummulitidae) für hohe Siedlungsdichte und Karbonatproduktion. Verh. dt. zool. Ges. 65: 289–292
Röttger, R. (1972b). Die Kultur vonHeterostegina depressa (Foraminifera: Nummulitidae). Mar. Biol. 15: 150–159
Röttger, R. (1976). Ecological observations ofHeterostegina depressa (Foraminifera, Nummulitidae) in the laboratory and in its natural habitat. Marit. Sediments (Spec. Publ. 1): 75–80
Röttger, R., Berger, W. H. (1972). Benthic Foraminifera: morphology and growth in clone cultures ofHeterostegina depressa. Mar. Biol. 15: 89–94
Röttger, R., Fladung, M., Schmaljohann, R., Spindler, M., Zacharias, H. (1986). A new hypothesis: the so-called megalospheric schizont of the larger foraminifera,Heterostegina depressa d'Orbigny 1826, in a separate species. J. foraml Res. 16: 141–149
Röttger, R., Irwan, A., Schmaljohann, R., Franzisket, L. (1980). Growth of the symbiont-bearing foraminiferaAmphistegina lobifera andHeterostegina depressa. In: Schwemmler, W., Schenk, H. E. A. (eds.) Endocytobiology, endosymbiosis and cell biology, Vol. 1. Walter de Gruyther, Berlin, p. 125–132
Valiela, I. (1984). Marine ecological processes. Springer-Verlag, New York
Zohary, T., Reiss, Z., Hottinger, L. (1980). Population dynamics ofAmphisorus hemprichii (foraminifera) in the Gulf of Elat (Aquaba), Red Sea. Ecologae geol. Helv. 73(3): 1071–1094
Author information
Authors and Affiliations
Additional information
Communicated by J. Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Lee, J.J., Sang, K., ter Kuile, B. et al. Nutritional and related experiments on laboratory maintenance of three species of symbiont-bearing, large foraminifera. Mar. Biol. 109, 417–425 (1991). https://doi.org/10.1007/BF01313507
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01313507