Summary
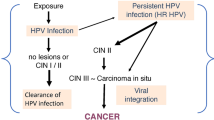
Human papillomaviruses (HPVs) are associated with at least 80% of cervical carcinomas and are classified as high-risk or low-risk based on whether or not they are commonly found in cervical cancers. The high-risk HPVs have early gene products (E6 and E7) that immortalize human keratinocytes and are at least partially responsible for causing cervical carcinoma. E6 and E7 from the high-risk viruses interact strongly with the tumor suppressors p53 and Rb; those from the low-risk HPVs do not. Transformation involves a multi-step process and requires additional factors besides high-risk HPV infection. High-risk HPVs are capable of immortalizing primary human keratinocytes in tissue culture, but such cells become transformed only after certain chromosomal changes take place, possibly having to do with oncogene activation. The DNA of high-risk HPVs is frequently (if not always) integrated into the genome of cancer cells; it is normally episomal in premalignant lesions. Integration disrupts the E2 and E5 genes and viral gene regulation. Cells containing integrated viral DNA show excessively high levels of E6 and E7. While there is some conflicting evidence, it appears that the p53 and Rb tumor-suppressor genes are more frequently mutated in HPV-negative tumors than they are in HPV-positive tumors, suggesting that for tumor formation to proceed the p53 and Rb proteins must be inactivated either by interaction with the viral proteins or by mutation. The presence of an activated oncogene in a cell lacking functional p53 or Rb may then be sufficient to cause tumor progression.
Similar content being viewed by others
References
Baker CC, Phelps WC, Lindgren V, Braun MJ, Gonda MA, Howley PM (1987) Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol 61: 962–971
Barak Y, Juven T, Haffner R, Oren M (1993) mdm2 expression is induced by wild type p53 activity. EMBO J 12: 461–468
Bedell MA, Hudson JB, Golub TR, Turyk ME, Hosken M, Wilbanks GD, Laimins LA (1991) Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation. J Virol 65: 2254–2260
Bedell MA, Jones KH, Grossman SR, Laimins LA (1989) Identification of human papillomavirus type 18 transforming genes in immortalized and primary cells. J Virol 63: 1247–1255
Benedict WF, Xu HJ, Takahashi R (1990) The retinoblastoma gene: its role in human malignancies. Cancer Invest 8: 535–540
Bookstein R, Lee WH (1991) Molecular genetics of the retinoblastoma suppressor gene. Crit Rev Oncog 2: 211–227
Braun L, Dürst M, Mikumo R, Gruppuso P (1990) Differential response of nontumorigenic and tumorigenic human papillomavirus type 16-positive epithelial cells to transforming growth factor beta 1. Cancer Res 50: 7324–7332
Chesters PM, McCance DJ (1989) Human papillomavirus types 6 and 16 in cooperation with Ha-ras transform secondary rat embryo fibroblasts. J Gen Virol 70: 353–365
Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH (1993) Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature 362: 849–852
Couturier J, Sastre-Garau X, Schneider-Maunoury S, Labib A, Orth G (1991) Integration of papillomavirus DNA near myc genes in genital carcinomas and its consequences for proto-oncogene expression. J Virol 65: 4534–4538
Cullen AP, Reid R, Campion M, Lorincz AT (1991) Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol 65: 606–612
Davies R, Hicks R, Crook T, Morris J, Vousden K (1993) Human papillomavirus type 16 E7 associates with a histone H1 kinase and with p107 through sequences necessary for transformation. J Virol 67: 2521–2528
DeCaprio JA, Ludlow JW, Lynch D, Furukawa Y, Griffin J, Piwnica-Worms H, Huang CM, Livingston DM (1989) The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell 58: 1085–1095
DiPaolo JA, Woodworth CD, Popescu NC, Notario V, Doniger J (1989) Induction of human cervical squamous cell carcinoma by sequential transfection with human papillomavirus 16 DNA and viral Harvey ras. Oncogene 4: 395–399
Dürst M, Gallahan D, Jay G, Rhim JS (1989) Glucocorticoid-enhanced neoplastic transformation of human keratinocytes by human papillomavirus type 16 and an activated ras oncogene. Virology 173: 767–771
Dyson N, Howley PM, Münger K, Harlow E (1989) The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243: 943–937
Farmer G, Bargonetti J, Zhu H, Friedman P, Prywes R, Prives C (1992) Wild-type p53 activates transcription in vitro. Nature 358: 83–86
Fuchs PG, Girardi F, Pfister H (1989) Human papillomavirus 16 DNA in cervical cancers and in lymph nodes of cervical cancer patients: a diagnostic marker for early metastases? Int J Cancer 43: 41–44
Fujita M, Inoue M, Tanizawa O, Iwamoto S, Enomoto T (1992) Alterations of the p53 gene in human primary cervical carcinoma with and without human papillomavirus infection. Cancer Res 52: 5323–5328
Heck DV, Yee CL, Howley PM, Münger K (1992) Efficiency of binding the retinoblastoma protein correlates with the transforming capacity of the E7 oncoproteins of the human papillomaviruses. Proc Natl Acad Sci USA 89: 4442–4446
Huibregtse JM, Scheffner M, Howley PM (1993) Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol Cell Biol 13: 775–784
Hurlin PJ, Kaur, P, Smith PP, Perez-Reyes N, Blanton RA, McDougall JK (1991) Progression of human papillomavirus type 18-immortalized human keratinocytes to a malignant phenotype. Proc Natl Acad Sci USA 88: 570–574
Imai Y, Matsushima Y, Sugimura T, Terada M (1991) Purification and characterization of human papillomavirus type 16 E7 protein with preferential binding capacity to the underphosphorylated form of retinoblastoma gene product. J Virol 65: 4966–4972
Kessis TD, Slebos RJ, Nelson WG, Kastan MB, Plunkett BS, Han SM, Lorincz AT, Hedrick L, Cho KR (1993) Human papillomavirus 16 E6 expression disrupts the p53-mediated cellular response to DNA damage. Proc Natl Acad Sci USA 90: 3988–3992
Koulos JP, Wright TC, Mitchell MF, Silva E, Atkinson EN, Richart RM (1993) Relationships between c-Ki-ras mutations, HPV types, and prognostic indicators in invasive endocervical adenocarcinomas, Gynecol Oncol 48: 364–369
Laiho M, DeCaprio JA, Ludlow JW, Livingston DM, Massague J (1990) Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell 62: 175–185
Langen R, Schweins T, Warshel A (1992) On the mechanism of guanosine triphosphate hydrolysis in ras p21 proteins. Biochemistry 31: 8691–8696
Lazo PA, Gallego MI, Ballester S, Feduchi E (1992) Genetic alterations by human papillomaviruses in oncogenesis. FEBS Lett 300: 109–113
Leechanachai P, Banks L, Moreau F, Matlashewski G (1992) The E5 gene from human papillomavirus type 16 is an oncogene which enhances growth factor-mediated signal transduction to the nucleus. Oncogene 7: 19–25
Lorincz AT, Reid R, Jenson AB, Greenberg MD, Lancaster W, Kurman RJ (1992) Human papillomavirus infection of the cervix: relative risk associations of 15 common anogenital types. Obstet Gynecol 79: 328–337
Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T (1993) p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362: 847–849
Matsukura T, Koi S, Sugase M (1989) Both episomal and integrated forms of human papillomavirus type 16 are involved in invasive cervical cancers. Virology 172: 63–72
Mihara K, Cao XR, Yen A, Chandler S, Driscoll B, Murphree AL, T'Ang A, Fung YK (1989) Cell cycle-dependent regulation of phosphorylation of the human retinoblastoma gene product. Science 246: 1300–1303
Moodie SA, Willumsen BM, Weber MJ, Wolfman A (1993) Complexes of Ras. GTP with Raf-1 and mitogen-activated protein kinase. Science 260: 1658–1661
Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM (1989) Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J 8: 4099–4105
Murphy CS, Pietenpol JA, Münger K, Howley PM, Moses HL (1991) c-myc and pRB: role in TGF-beta 1 inhibition of keratinocyte proliferation. Cold Spring Harb Symp Quant Biol 56: 129–135
Neel BG, Jhanwar SC, Chaganti RS, Hayward WS (1982) Two human c-onc genes are located on the long arm of chromosome 8. Proc Natl Acad Sci USA 79: 7842–7846
Nishikawa T, Yamashita T, Yamada T, Kobayashi H, Ohkawara A, Fujinaga K (1991) Tumorigenic transformation of primary rat embryonal fibroblasts by human papillomavirus type 8 E7 gene in collaboration with the activated H-ras gene. Jpn J Cancer Res 82: 1340–1343
Pagano M, Dürst M, Joswig S, Draetta G, Jansen-Dürr P (1992) Binding of the human E2F transcription factor to the retinoblastoma protein but not to cyclin A is abolished in HPV-16-immortalized cells. Oncogene 7: 1681–1686
Pelisson I, Soler C, Pechoux C, Chignol MC, Viac J, Euvrard S, Chardonnet Y (1992) c-myc and c-Ha-ras cellular oncogenes and human papillomaviruses in benign and malignant cutaneous lesions. J Dermatol Sci 3: 56–67
Piccione E, Case RD, Domchek SM, Hu P, Chaudhuri M, Backer JM, Schlessinger J, Shoelson SE (1993) Phosphatidylinositol 3-kinase p85 SH2 domain specifictiy defined by direct phosphopeptide/SH2 domain binding. Biochemistry 32: 3197–3202
Pietenpol JA, Stein RW, Moran E, Yaciuk P, Schlegel R, Lyons RM, Pittelkow MR, Münger K, Howley PM, Moses HL (1990) TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell 61: 777–785
Pim D, Collins M, Banks L (1992) Human papillomavirus type 16 E5 gene stimulates the transforming activity of the epidermal growth factor receptor. Oncogene 7: 27–32
Popescu NC, Zimonjic D, DiPaolo JA (1990) Viral integration, fragile sites, and proto-oncogenes in human neoplasia. Hum Gen 84: 383–386
Prive GG, Milburn MV, Tong L, de Vos AM, Yamaizumi Z, Nishimura S, Kim SH (1992) X-ray crystal structures of transforming p21 ras mutants suggest a transition-state stabilization mechanism for GTP hydrolysis. Proc Natl Acad Sci USA 89: 3649–3653
Reeves WC, Brinton LA, Garcia M, Brenes MM, Herrero R, Gaitan E, Tenorio F, de Britton RC, Rawls WE (1989) Human papillomavirus infection and cervical cancer in Latin America. N Engl J Med 320: 1437–1441
Reifenberger G, Liu L, Ichimura K, Schmidt EE, Collins VP (1993) Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res 53: 2736–2739
Riou G, Barrois M, Sheng ZM, Duvillard P, Lhomme C (1988) Somatic deletions and mutations of c-Ha-ras gene in human cervical cancers. Oncogene 3: 329–333
Riou G, Barrios M, Tordjman I, Dutronquay V, Orth G (1984) Presence of papil-lomavirus genomes and amplification of the c-myc and C-Has-ras oncogenes in invasive cancers of the uterine cervix. CR Acad Sci III 299: 575–580
Romanczuk H, Howley PM (1992) Disruption of either the E1 or the E2 regulatory gene of human papillomavirus type 16 increases viral immortalization capacity. Proc Natl Acad Sci USA 89: 3159–3163
Romanczuk H, Villa LL, Schlegel R, Howley PM (1991) The viral transcriptional regulatory region upstream of the E6 and E7 genes is a major determinant of the differential immortalization activities of human papillomavirus types 16 and 18. J Virol 65: 2739–2744
Sang BC, Barbosa MS (1992) Single amino acid substitutions in “low-risk” human papillomavirus (HPV) types 6 E7 protein enchance features characteristic of the “high-risk” HPV E7 oncoproteins. Proc Natl Acad Sci USA 89: 8063–8067
Sang BC, Barbosa MS (1992) Increased E6/E7 transcription in HPV 18-immortalized human keratinocytes results from inactivation of E2 and additional cellular events. Virology 189: 448–455
Scheffner M, Münger K, Byrne JC, Howley PM (1991) The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci USA 88: 5523–5527
Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM (1990) The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63: 1129–1136
Schwab M, Varmus HE, Bishop JM, Grzeschik KH, Naylor SL, Sakaguchi AY, Brodeur G, Trent J (1984) Chromosome localization in normal human cells and neuroblastomas of a gene related to c-myc. Nature 308: 288–291
Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, Zur Hausen H (1985) Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 314: 111–114
Schwarz JK, Devoto SH, Smith EJ, Chellappan SP, Jakoi I, Nevins JR (1993) Interactions of the p107 and Rb proteins with E2F during the cell proliferation response. EMBO J 12: 1013–1020
Seth A, Alvarez E, Gupta S, Davis RJ (1991) A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression. J Biol Chem 266: 23521–23524
Straight SW, Hinkle PM, Jewers RJ, McCance DJ (1993) The E5 oncoprotein of human papillomavirus type 16 transforms fibroblasts and effects the downregulation of the epidermal growth factor receptor in keratinocytes. J Virol 67: 4521–4532
Strong LC, Williams WR, Tainsky MA (1992) The Li-Fraumeni syndrome: from clinical epidemiology to molecular genetics. Am J Epidemiol 135: 190–199
von Knebel-Doeberitz M (1990) The role of papilliomaviruses in the etiology of cervix cancer. Geburtshilfe Frauenheilkd 50: 511–517
Wasylyk B, Hahn SL, Giovane A (1993) The Ets family of transcription factors. Eur J Biochem 211: 7–18
Werness BA, Levine AJ, Howley PM (1990) Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248: 76–79
Whyte P, Buchkovich KJ, Horowitz JM, Friend SH, Raybuck M, Weinberg RA, Harlow E (1988) Association between on oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature 334: 124–129
Willis G, Jennings B, Ball RY, New NE, Gibson I (1993) Analysis of ras point mutations and human papillomavirus 16 and 18 in cervical carcinomata and their metastases. Gynecol Oncol 49: 359–364
Yasumoto S, Burkhardt AL, Doniger J, DiPaolo JA (1986) Human papillomavirus type 16 DNA-induced malignant transformation of NIH 3T3 cells. J Virol 57: 572–577
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Swan, D.C., Vernon, S.D. & Icenogle, J.P. Cellular proteins involved in papillomavirus-induced transformation. Archives of Virology 138, 105–115 (1994). https://doi.org/10.1007/BF01310042
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01310042