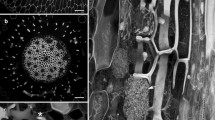
Summary
Premitotic stages of experimentally induced vascular differentiation were investigated by confocal laser scanning-fluorescence and electron microscopy. Activation of former quiescent and highly vacuolated cortex cells, observed in living and in fixed tissue, involves a centripetal movement of the nucleus along newly-formed, thick cytoplasmic strands. These traverse the large vacuoles with angles different from the former polar axis, thereby anticipating the orientation of later developing phragmosomes, future divisions, and the course of wound-vascular elements.
A premitotic increase in cellular activity is reflected by the number of mitochondria, rough ER-cisternae, active dictyosomes and dense cytoplasm as well as by shape of nuclei and development of “nucleolar vacuoles”. Microtubules occur in parallel arrays running through the transvacuolar strands and radiating from the nucleus towards the cell wall. They might be responsible for the centripetal movement of the nucleus through the vacuole and/or might participate on the orientation of the plane of a future division. Preprophase bands were not detected in cortex cells of the investigated root region, which prior to wounding were already fully elongated.
Similar content being viewed by others
Abbreviations
- DiOC:
-
3,3′-dihexyloxacarbocyanine iodide
- LSM:
-
confocal laserscanning microscope
- MSB:
-
microtubule-stabilizing buffer
References
Aloni R, Wolf A (1984) Suppressed buds embedded in the bark across the bole and the occurrence of their circular vessels inFicus religiosa. Am J Bot 71: 1060–1066
Bakhuizen R, Spronsen PC van, Sluiman-den Hertog FAJ, Venverloo CJ, Goosen-de Roo L (1985) Nuclear envelope radiating microtubules in plant cells during interphase mitosis transition. Protoplasma 128: 43–51
Behnke H-D, Schulz A (1980) Fine structure, pattern of division, and course of wound phloem inColeus blumei. Planta 150: 357–365
Gersani M (1985) Appearance of new transport capacity in wounded plants. J Exp Bot 36: 1809–1816
Goosen-de Roo L, Bakhuizen R, Spronsen PC van, Libbenga KR (1984) The presence of extended phragmosomes containing cytoskeletal elements in fusiform cambial cells ofFraxinus excelsior L. Protoplasma 122: 145–152
Gunning BES (1982) The cytokinetic apparatus: its development and spatial regulation. In:Lloyd (ed) The cytoskeleton. Academic Press, London, pp 229–292
Hardham AR, McCully M (1982 a) Reprogramming of cells following wounding in pea (Pisum sativum L.) roots. I. Cell division and differentiation of new vascular elements. Protoplasma 112: 143–151
— (1982 b) Reprogramming of cells following wounding in pea (Pisum sativum L.) roots. II. The effects of caffeine and colchicine on the development of new vascular elements. Protoplasma 112: 152–166
Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Techn 45: 115–120
Karnovski MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27: 137A-138A
Kirschner H, Sachs T (1978) Cytoplasmic reorientation: An early stage of vascular differentiation. Isr J Bot 27: 131–137
Matzke MA, Matzke AJM (1986) Visualization of mitochondria and nuclei in living plant cells by the use of a potential-sensitive fluorescent dye. Plant Cell Envir 9: 73–77
Pickett-Heaps JD, Northcote DH (1966) Cell division in the formation of the stomatal complex of the young leaves of wheat. J Cell Sci 1: 121–128
Quader H, Schnepf E (1986) Endoplasmic reticulum and cytoplasmic streaming: Fluorescence microscopical observations in adaxial epidermis cells of onion bulb scales. Protoplasma 131: 250–252
Quader H, Hofmann A, Schnepf E (1987) Shape and movement of the endoplasmic reticulum in onion bulb epidermis cells: possible involvement of actin. Eur J Cell Biol 44: 17–26
Rana MA, Gahan PB (1983) A quantitative cytochemical study of determination for xylem-element formation in response to wounding in roots ofPisum sativum L. Planta 157: 307–316
Robbertse PJ, McCully M (1979) Regeneration of vascular tissue in wounded pea roots. Planta 145: 167–173
Sachs T (1975) The induction of transport channels by auxin. Planta 127: 201–206
— (1981) Polarity changes and tissue organization in plants. In:Schweiger HG (ed) International cell biology 1980/81. Springer, Heidelberg, pp 489–496
— (1984) Axiality and polarity in vascular plants. In:Barlow PW, Carr DJ (eds) Positional controls in plant development. Cambridge University Press, Cambridge New York New Rochelle Melbourne Sydney, pp 193–224
—,Cohen D (1982) Circular vessels and the control of vascular differentiation in plants. Differentiation 21: 22–26
Schmiedel G, Schnepf E (1979) Side branch formation and orientation in the caulonema of the moss,Funaria hygrometrica: Normal development and fine structure. Protoplasma 100, 367–383
Schnepf E, Traitteur R von (1973) Über die traumatotaktische Bewegung der Zellkerne inTradescantia-Blättern. Z Pflanzenphysiol 69: 181–184
Schulz A (1986) Wound phloem in transition to bundle phloem in primary roots ofPisum sativum L.: I. Development of bundle-leaving wound-sieve tubes. Protoplasma 130: 12–26
— (1987) Sieve element differentiation and fluoresceine translocation in wound-phloem of pea roots after complete severance of the stele. Planta 170: 289–299
Sinnott EW, Bloch R (1941) Division in vacuolate plant cells. Am J Bot 28: 225–232
Venverloo CJ, Hovenkamp PH, Weeda AJ, Libbenga KR (1980) Cell divisions inNautilocalyx explants I. Phragmosome, preprophase band and plane of division. Z Pflanzenphysiol 100: 161–174
White JG, Amos WB, Fordham M (1987) An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol 105: 41–48
Wick SM, Duniec J (1983): Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. I. Preprophase band development and concomitant appearance of nuclear envelope-associated tubulin. J Cell Biol 97: 235–243
— (1984) Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. II. Transition between Preprophase band and the mitotic spindle. Protoplasma 122: 45–55
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schulz, A. Vascular differentiation in the root cortex of peas: Premitotic stages of cytoplasmic reactivation. Protoplasma 143, 176–187 (1988). https://doi.org/10.1007/BF01291162
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01291162