Summary
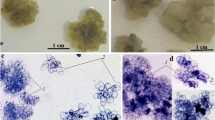
Suspension cultures were initiated from somatic embryos and embryogenic callus ofDactylis glomerata L. in SH-30 liquid medium [Schenk andHildebrandt (1972) containing 30 μM 3,6-dichloro-o-anisic acid (dicamba)] with or without 1.5 gl−1 casein hydrolysate. Established suspension cultures maintained in SH-30 without casein hydrolysate proliferated when cell masses underwent cell division and enlargement. These cultures contained numerous root primordia and increased in volume when the cell masses continued to grow and fragment. Embryos developed only when cell masses were plated on solidified SH-30 medium. Cultures maintained in SH-30 liquid medium with casein hydrolysate also proliferated by the growth and fragmentation of cell masses. However, these cell masses contained numerous developing embryos and possessed few or no root primordia. Embryos were either attached to cell masses by a suspensor-like structure or were free and became fully developed in the liquid medium. Newly formed embryos became callused and produced embryogenic cell masses. Embryos germinated either in liquid or on solid SH medium without dicamba. The resulting plantlets possessed green shoots and well developed roots. Plants from suspension and suspension-derived callus cultures have been established in soil and grown to maturity.
Similar content being viewed by others
References
Brown, W. V., 1960: The morphology of the grass embryo. Phytomorphology10, 215–223.
Conger, B. V., Carabia, J. V., 1978: Callus induction and plantlet regeneration in orchardgrass. Crop Sci.18, 157–159.
—,Hanning, G. E., Gray, D. J., McDaniel, J. K., 1983: Direct embryogenesis from mesophyll cells of orchardgrass. Science221, 850–851.
Conger, B. V., McDonnell, R. E., 1983: Plantlet formation from cultured inflorescence ofDactylis glomerata L. Plant Cell Tissue Organ Culture2, 191–197.
Conrad, P. A., Binari, L. L. W., Racusen, R. H., 1982: Rapidly-secreting oat cells serve as a model system for the study of cellular exocytosis. Characterization of cells and isolated secretory vesicles. Protoplasma112, 196–204.
Cure, W. W., Mott, R. L., 1978: A comparative anatomical study of organogenesis in cultured tissue of maize, wheat, and oats. Physiol. Plant.42, 91–96.
Dale, P. J., Thomas, E., Brettell, R. I. S., Wernicke, W., 1981: Embryogenesis from cultured immature inflorescences and nodes ofLolium multiflorum. Plant Cell Tissue Organ Culture1, 47–55.
Haccius, B., 1978: Question of unicellular origin of non-zygotic embryos in callus cultures. Phytomorphology28, 74–81.
Hanning, G. E., Conger, B. V., 1982: Embryoid and plantlet formation from leaf segments ofDactylis glomerata L. Theoret. appl. Genet.63, 155–159.
Haydu, Z., Vasil, I. K., 1981: Somatic embryogenesis and plant regeneration from leaf tissues and anthers ofPennisetum purpureum Schum. Theoret. appl. Genet.59, 269–273.
Heyser, J. W., Nabors, M. W., 1982 a: Long term plant regeneration, somatic embryogenesis and green spot formation in secondary oat (Avena sativa) callus. Z. Pflanzenphysiol.107, 153–160.
— —, 1982 b: Regeneration of proso millet from embryogenic calli derived from various plant parts. Crop Sci.22, 1070–1074.
Ho, W. J., Vasil, I. K., 1983: Somatic embryogenesis in sugarcane (Saccharum officinarum L.): Growth and plant regeneration from embryogenic cell suspension cultures. Ann. Bot.51, 719–726.
Kao, K. N., Gamborg, O. L., Michayluk, M. R., Keller, W. A., Miller, R. A., 1973: The effects of sugars and inorganic salts on cell regeneration and sustained division in plant protoplasts. Colloq. Int. C.N.R.S.212, 207–213.
King, P. J., Potrykus, I., Thomas, E., 1978:In vitro genetics of cereals: problems and perspectives. Physiol. Veg.16, 381–399.
Lu, C., Vasil, I. K., 1981 a: Somatic embryogenesis and plant regeneration from leaf tissues ofPanicum maximum Jacq. Theoret. appl. Genet.59, 275–280.
— —, 1981 b: Somatic embryogenesis and plant regeneration from freely-suspended cells and cell groups ofPanicum maximum Jacq. Ann. Bot.48, 543–548.
— —,Ozias-Akins, P., 1982: Somatic embryogenesis inZea mays L. Theoret. appl. Genet.62, 109–112.
—,Vasil, V., Vasil, I. K., 1981: Isolation and culture of protoplasts ofPanicum maximum Jacq. (Guinea grass): Somatic embryogenesis and plantlet formation. Z. Pflanzenphysiol.104, 311–318.
McDaniel, J. K., Conger, B. V., Graham, E. T., 1982: A histological study of tissue proliferation, embryogenesis, and organogenesis from tissue cultures ofDactylis glomerata L. Protoplasma110, 121–128.
Mott, R. L., Cure, W. W., 1978: Anatomy of maize tissue cultures. Physiol. Plant.42, 139–145.
Rangan, T. S., Vasil, I. K., 1983: somatic embryogenesis and plant regeneration in tissue cultures ofPanicum miliaceum L. andPanicum miliare Lamk. Z. Pflanzenphysiol.109, 49–53.
Schenk, R. U., Hildebrandt, A. C., 1972: Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot.50, 199–204.
Syono, K., 1965: Changes in organ forming capacity of carrot root callus during subculture. Plant and Cell Physiol.65, 403–419.
Thomas, E., King, P. J., Potrykus, I., 1977: Shoot and embryo-like structure formation from cultured tissues ofSorghum bicolor. Naturwiss.64, 587.
Vasil, V., Vasil, I. K., 1980: Isolation and culture of cereal protoplasts. II. Embryogenesis and plantlet regeneration from protoplasts ofPennisetum americanum. Theoret. appl. Genet.56, 97–99.
— —, 1981 a: Somatic embryogenesis and plant regeneration from suspension cultures of pearl millet (Pennisetum americanum). Ann. Bot.47, 669–678.
— —, 1981 b: Somatic embryogenesis and plant regeneration from tissue cultures ofPennisetum americanum andP. americanum P. purpureum hybrid. Amer. J. Bot.68, 864–872.
— —, 1982 a: Characterization of an embryogenic cell suspension culture derived from cultured inflorescences ofPennisetum americanum (pearl millet,Gramineae). Amer. J. Bot.69, 1411–1449.
— —, 1982 b: The ontogeny of somatic embryos ofPennisetum americanum (L.) K. Schum. I. In cultured immature embryos. Bot. Gaz.143, 454–465.
Wang, D., Vasil, I. K., 1982: Somatic embryogenesis and plant regeneration from inflorescence segments ofPennisetum purpureum Schum. (Napier or elephant grass). Plant Sci. Lett.25, 147–154.
Wernicke, W., Brettell, R., 1980: Somatic embryogenesis inSorghum bicolor leaves. Nature (Lond.)287, 138–139.
— —,Wakizuka, T., Potrykus, I., 1981: Adventitious embryoid and root formation from rice leaves. Z. Pflanzenphysiol.103, 361–365.
—,Potrykus, I., Thomas, E., 1982: Morphogenesis from cultured leaf tissue ofSorghum bicolor—The morphogenetic pathways. Protoplasma111, 53–66.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gray, D.J., Conger, B.V. & Hanning, G.E. Somatic embryogenesis in suspension and suspension-derived callus cultures ofDactylis glomerata . Protoplasma 122, 196–202 (1984). https://doi.org/10.1007/BF01281697
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01281697