Summary
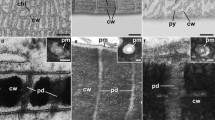
Cell plate formation in Chara zeylanica was compared with recent models of cytokinesis in higher plants in order to gain insight into the evolutionary origin of plant cytokinetic processes. Transmission electron microscopy (TEM) reveals that while cytokinesis in C. zeylanica bears many features in common with that in higher plants, there are significant differences. Unlike that in higher plants, cytokinesis in C. zeylanica begins with a congregation of smooth membrane tubules that are closely associated with endoplasmic reticulum (ER) and Golgi membranes. Mitochondria and other organelles excluded by the phragmoplast in higher plants are present as well. Unlike in higher plants, phragmoplast microtubules persist throughout cytokinesis in C. zeylanica, and the cell plate generally forms across the whole cell at once, though development is patchy, due to small regions developing at different rates; the ends of the plate form last. By identifying aspects of cytokinesis that are different in C. zeylanica and plants, our study indicates which cytokinetic features are more likely to be derived, and which are more likely to be ancestral. In addition, we demonstrated that all nodal cells of C. zeylanica are interconnected via plasmodesmata, lending support to the idea that, while Chara spp. are generally considered to be filamentous organisms, nodal regions may be thought of as meristemlike tissues.
Similar content being viewed by others
Abbreviations
- HPF:
-
high-pressure freezing
- KFe:
-
potassium ferricyanide
- SCF:
-
stepwise chemical fixation
- TEM:
-
transmission electron microscopy
References
Bold HC, Wynne MJ (1985) Introduction to the algae, 2nd edn. Prentice-Hall, Englewood Cliffs, NJ
Brown RC, Lemmon BE, Graham LE (1994) Morphogenetic plastid migration and microtubule arrays in mitosis and cytokinesis in the green alga Coleochaete orbicularis. Am J Bot 81: 127–133
Cook ME, Graham LE, Botha CEJ, Lavin CA (1997) Comparative ultrastructure of plasmodesmata of Chara and selected bryophytes: toward an elucidation of the evolutionary origin of plant plasmodesmata. Am J Bot 84: 1169–1178
Cronshaw J, Esau K (1968) Cell division in leaves of Nicotiana. Protoplasma 65: 1–24
Cutter EG, Hung CY (1972) Symmetric and asymmetric mitosis and cytokinesis in the root tip of Hydrocharis morsus-ranae L. J Cell Sci 11: 723–737
Ding B, Turgeon R, Parthasarathy MV (1992) Substructure of freeze-substituted plasmodesmata. Protoplasma 169: 28–41
Evert RF, Deshpande BP (1970) An ultrastructural study of cell division in the cambium. Am J Bot 57: 942–961
Frame P, Sawa T (1975) Comparative anatomy of Charophyta II: the axial nodal complex — an approach to the taxonomy of Lamprothamnion. J Phycol 11: 202–205
Franceschi VR, Ding B, Lucas WJ (1994) Mechanism of plasmodesmata formation in characean algae in relation to evolution of intercellular communication in higher plants. Planta 192: 347–358
Fritsch FE (1935) The structure and reproduction of the algae, vol 1. Macmillan, New York
Graham LE (1993) Origin of land plants. Wiley, New York
— (1996) Green algae to land plants: an evolutionary transition. J Plant Res 109: 241–251
—, Kaneko Y (1991) Subcellular structures of relevance to the origin of land plants (embryophytes) from green algae. Crit Rev Plant Sci 10: 323–342
—, Delwiche CF, Mishler B (1991) Phylogenetic connections between the “green algae” and the “bryophytes”. Adv Bryol 4: 213–244
Gunning BES (1982) The cytokinetic apparatus: its development and spatial regulation. In: Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 229–292
Hawes CR, Juniper BE, Horne JC (1981) Low and high voltage electron microscopy of mitosis and cytokinesis in maize roots. Planta 152: 397–407
Hepler PK (1981) The structure of the endoplasmic reticulum revealed by osmium tetroxide potassium ferricyanide staining. Eur J Cell Biol 26: 102–110
— (1982) Endoplasmic reticulum in the formation of the cell plate and plasmodesmata. Protoplasma 111: 121–133
—, Newcomb EH (1967) Fine structure of cell plate formation in the apical meristem of Phaseolus roots. J Ultrastruct Res 19: 498–513
Kaplan DR, Hagemann W (1991) The relationship of cell and organism in vascular plants. BioScience 41.: 693–703
Manhardt JR (1994) Phylogenetic analysis of green plant rbcL sequences. Mol Phylogenet Evol 3: 114–127
McCourt RM, Karol KG, Guerlesquin M, Feist M (1996) Phytogeny of extant genera in the family Characeae (Charales, Charophyceae) based on rbcL sequences and morphology. Am J Bot 83: 125–131
Mishler BD, Lewis LA, Buchheim MA, Renzaglia KS, Garbary DJ, Delwiche CF, Zechman FW, Kantz TS, Chapman RL (1994) Phylogenetic relationships of the “green algae” and “bryophytes”. Ann Missouri Bot Garden 81: 451–483
Pickett-Heaps JD (1967a) Ultrastructure and differentiation in Chara sp. I: vegetative cells. Aust J Biol Sci 20: 539–551
— (1967b) Ultrastructure and differentiation in Chara sp. II: mitosis. Aust J Biol Sci 20: 883–894
— (1975) Green algae. Sinauer Associates, Sunderland, Mass
Porter KR, Machado RD (1997) Studies on the endoplasmic reticulum IV: its form and distribution during mitosis in cells of onion root tip. J Biophys Biochem Cytol 7: 167–180
Samuels AL, Giddings TH Jr, Staehelin LA (1995) Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol 130: 1345–1357
Staehelin LA, Hepler, PK (1996) Cytokinesis in higher plants. Cell 84: 821–824
Sundaralingam VS (1954) The developmental morphology of Chara zeylanica. J Ind Bot Soc 33: 272–297
Taylor DP (1988) Direct measurement of the osmotic effects of buffers and fixatives in Nitella flexilis. J Microsc 150: 71–80
Whaley WG, Dauwalder M, Kephart JE (1966) The Golgi apparatus and an early stage of cell plate formation. J Ultrastruct Res 15: 169–180
Wood RD (1967) Charophytes of North America. Stella's Printing, West Kingston
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cook, M.E., Graham, L.E. & Lavin, C.A. Cytokinesis and nodal anatomy in the charophycean green alga Chara zeylanica . Protoplasma 203, 65–74 (1998). https://doi.org/10.1007/BF01280588
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01280588