Summary
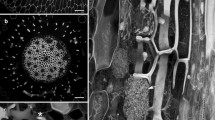
We describe the use of scanning electron microscopy to provide novel views of the three-dimensional morphology of the ingrowth wall in epidermal transfer cells of cotyledons of developingVicia faba seed. Wall ingrowth deposition in these cells amplifies the surface area of plasma membrane available for transport of solutes during cotyledon development. Despite the physiological importance of such amplification, little is known about wall ingrowth morphology and deposition in transfer cells. A detailed morphological analysis of wall deposition in this study clearly established for the first time that wall ingrowths are deposited at scattered, discrete loci as papillate ingrowth projections. The new views of the ingrowth wall revealed that these projections branch and fuse laterally, and fusion occurs by fine connections to form a fenestrated sheet or layer. This sheet of wall material then provides a base for further deposition of ingrowth projections to progressively build many interconnected, fenestrated layers. Consolidation, or filling-in, of the fenestrae in these layers appears to occur from small fingerlike protrusions of wall material which extend laterally from the most recently deposited surface of the fenestrae. We propose that deposition of fenestrated layers may provide a mechanism for maintaining continuous amplification of plasma membrane surface area in the face of turnover of the plasma membrane and transporter proteins associated with it. The techniques reported in this paper will provide new opportunities to investigate wall ingrowth deposition and its regulation in transfer cells.
Similar content being viewed by others
Abbreviations
- SEM:
-
scanning electron microscopy
- TEM:
-
transmission electron microscopy
References
Apostolakos P, Galatis B (1998) Probable involvement of cytoskeleton in stomatal-pore formation inAsplenium nidus L. Protoplasma 203: 48–57
— — (1999) Microtubule and actin filament organization during stomatal morphogenesis in the fernAsplenium nidus II: guard cells. New Phytol 141: 209–223
Bagnall N, Wang X-D, Scofield GN, Furbank RT, Offler CE, Patrick JW (2000) Sucrose transport-related genes are expressed in both maternal and filial tissues of developing wheat grains. Aust J Plant Physiol 27: 1009–1020
Bonnemain JL, Borquin S, Renault S, Offler CE, Fisher DG (1991) Transfer cells: structure and physiology. In: Bonnemain L, Delrot S (eds) Recent advances in phloem transport and assimilate compartmentation. Ouest Editions, Nantes, pp 74–83
Briarty LG (1971) A method for preparing living plant cell walls for scanning electron microscopy. J Microsc 94: 181–183
Brisson JD, Peterson RL (1975) SEM of fractured plant material embedded in glycol methacrylate. Microsc Soc Can II: 64–65
Bulbert MW, Offler CE, McCurdy DW (1998) Polarized microtubule deposition coincides with wall ingrowth formation in transfer cells ofVicia faba L. cotyledons. Protoplasma 201: 8–16
Burgoyne RD, Morgan A (1993) Regulated exocytosis. Biochem J 293: 305–316
Chaffey NJ, Barlow PW, Barnett JR (2000) A cytoskeletal basis for wood formation in angiosperm trees: the involvement of micro-filaments. Planta 210: 890–896
Chambers TC, Hamilton CD (1973) Scanning electron microscopy of transfer cells: a new method for preparation of plant tissues. J Microsc 99: 65–68
Dashek MV, Thomas HR, Rosem WG (1971) Secretory cells of lily pistil II: electron microscope cytochemistry of canal cells. Am J Bot 58: 909–920
deCamilli PD, Jahn R (1990) Pathways to regulated exocytosis in neurons. Annu Rev Physiol 52: 625–645
DeWitt G, Richards J, Mohnen D, Jones AM (1999) Comparative compositional analysis of walls with two different morphologies: archetypical versus transfer-cell like. Protoplasma 209: 238–245
Eleftheriou EP (1994) Abnormal structure of protophloem sieve-element cell wall in colchicine-treated roots ofTriticum aestivum L. Planta 193: 266–274
Farley SJ, Patrick JW, Offler CE (2000) Functional transfer cells differentiate in cultured cotyledons ofVicia faba L. seeds. Protoplasma 214: 102–117
Felker FC, Shannon JC (1980) Movement of14C-labeled assimilates into kernels ofZea mays L.: an anatomical examination and microautoradiographic study of assimilate transfer. Plant Physiol 65: 864–870
Fieuw S, Patrick JW (1993) Mechanism of photosynthate efflux fromVicia faba L. seed coats. J Exp Bot 44: 65–74
Fussell LK, Dwarte DM (1980) Structural changes of the grain associated with black region formation inPennisetum americanum. J Exp Bot 31: 645–654
Giddings TH Jr, Staehelin LA (1991) Microtubule-mediated control of microfibril deposition: a re-examination of the hypothesis. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 85–97
Gunning BES (1977) Transfer cells and their roles in transport of solutes in plants. Sci Prog Oxf 64: 539–568
—, Pate JS (1969) Transfer cells: plant cells with wall ingrowths, specialized in relation to short distance transport of solutes: their occurrence, structure and development. Protoplasma 68: 107–133
— — (1974) Transfer cells. In: Robards AW (ed) Dynamic aspects of plant ultrastructure. McGraw-Hill, London, pp 441–479
Harrington GN, Franceschi VR, Offler CE, Patrick JW, Tegeder M, Frommer WB, Harper JF, Hitz WD (1997a) Cell specific expression of three genes involved in plasma membrane sucrose transport in developingVicia faba seed. Protoplasma 197: 160–173
—, Nussbaumer Y, Wang X-D, Tegeder M, Franceschi VR, Frommer WB, Patrick JW, Offler CE (1997b) Spatial and temporal expression of sucrose transport-related genes in developing cotyledons ofVicia faba L. Protoplasma 200: 35–50
Hepler PK (1981) Morphogenesis of tracheary elements and guard cells. In: Kiermayer O (ed) Cytomorphogenesis in plants. Springer, Wien New York, pp 327–347 (Cell biology monographs, vol 8)
Hogetsu T (1991) Mechanism for formation of the secondary wall thickening in tracheary elements: microtubules and microfibrils of tracheary elements ofPisum sativum L. andCommelina communis L. and the effects of amiprophosmethyl. Planta 185: 190–200
Jones MGK, Dropkin VH (1976) Scanning electron microscopy of nematode-induced giant transfer cells. Cytobios 15: 149–161
—, Gunning BES (1976) Transfer cells and nematode induced giant cells inHelianthemum. Protoplasma 87: 273–279
Jung G, Wernicke W (1990) Cell shaping and microtubules in developing mesophyll of wheat. Protoplasma 153: 141–148
McDonald R, Fieuw S, Patrick JW (1996) Sugar uptake by the dermal transfer cells of developing cotyledons ofVicia faba L.: mechanism of energy coupling. Planta 198: 502–509
Muallem S, Kwiatkwoska K, Xu X, Yin HL (1995) Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol 128: 589–598
Offler CE, Patrick JW (1993) Pathway of photosynthate transfer in the developing seed ofVicia faba L.: a structural assessment of the role of transfer cells in unloading from the seed coat. J Exp Bot 44: 711–724
—, Liet E, Sutton EG (1997) Transfer cell induction in cotyledons ofVicia faba L. Protoplasma 200: 51–64
Patrick JW, Offler CE (1995) Post-sieve element transport of sucrose in developing seeds. Aust J Plant Physiol 22: 681–702
Rost TL, Izaguirre de Artucio P, Risely EB (1984) Transfer cells in the placental pad and caryopsis coat ofPappophorum subbulosum Arech. (Poaceae). Am J Bot 71: 948–957
Singh S, Lazzaro MD, Walles B (1999) Microtubule organization in the differentiating transfer cells of the placenta inLilium spp. Protoplasma 207: 75–83
Spungin B, Margalit I, Breitbart H (1995) Sperm exocytosis reconstructed in a cell-free system: evidence for the involvement of phospholipase C and actin filaments in membrane fusion. J Cell Sci 108: 2525–2535
Tegeder M, Wang X-D, Frommer WB, Offler CE, Patrick JW (1999) Sucrose transport into developing seeds ofPisum sativum L. Plant J 18: 151–161
—, Offler CE, Frommer WB, Patrick JW (2000) Amino acid transporters are localized to transfer cells of developing pea seeds. Plant Physiol 122: 319–325
Wang HL, Offler CE, Patrick JW (1994) Nucellar projection transfer cells in the developing wheat grain. Protoplasma 182: 39–52
— — — (1995a) The cellular pathway of photosynthate transfer in the developing wheat grain II: a structural analysis and histochemical study of the pathway from the crease phloem to the endosperm cavity. Plant Cell Environ 18: 373–388
—, Patrick JW, Offler CE, Wang X-D (1995b) The cellular pathway of photosynthate transfer in the developing wheat grain III: a structural analysis and physiological study of the pathway from the endosperm cavity to the starchy endosperm. Plant Cell Environ 18: 389–407
Yeung EC, Peterson RL (1974) Fine structure during ontogeny of xylem transfer cells in the rhizome ofHieracium floribundum. Can J Bot 53: 432–438
Zee S, O'Brien TP (1971) Aleurone transfer cells and other structural features of the spikelet of millet. Aust J Biol Sci 24: 391–395
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Brian E. S. Gunning on the occasion of his 65th birthday
Rights and permissions
About this article
Cite this article
Talbot, M.J., Franceschi, V.R., McCurdy, D.W. et al. Wall ingrowth architecture in epidermal transfer cells ofVicia faba cotyledons. Protoplasma 215, 191–203 (2001). https://doi.org/10.1007/BF01280314
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01280314