Summary
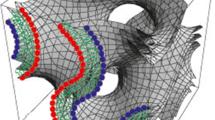
The “open” type of prolamellar body in etiplasts was examined by electron microscopy to characterise its three-dimensional organisation. As in more compact forms of prolamellar body, its basic geometric unit is a tetrahedrally branched tubule. In the “open” type, these lie smoothly confluent with one another at the vertices of 5- and 6-membered rings which circumscribe the faces of three kinds of polyhedra: pentagonal dodecahedra (with 12 pentagonal faces), 14-hedra (2 opposite hexagonal faces joined by two circlets of six pentagonal faces), and 15-hedra (3 hexagonal and 12 pentagonal faces). These polyhedra join confluently in their turn, sharing faces with one another in at least one recognisable superstructure which accounts for the appearance of “open” prolamellar bodies in many ultrathin sections. In this organisation, columns of pentagonal dodecahedra are arranged at 120 ° to one another in the x-y-plane of the lattice. They do not fill the plane but intersect so as to delimit voids in the form of hexagonally arranged 14-hedra (with hexagonal rings in the x-y-plane). Strata of this type alternate with strata made of face-sharing 15-hedra (with their hexagonal rings normal to x-y), which also delimit 14-hedra. The 14-hedra thus lie in register in the z-axis in hexagonally arranged columns, normal to the alternating strata. Other possible organisations cannot be excluded and local variations and dislocations certainly occur, but many micrographs that display elements of symmetry in “open” prolamellar bodies can be matched to thin slices through such a model. Its geometry is like that of the cages of water molecules in type IV (sensu Jeffrey=type IIIsensu O'Keeffe) clathrate-hydrates, point group P6/mmm, but about two orders of magnitude larger.
Similar content being viewed by others
References
Berry DR, Smith H (1971) Red-light stimulation of prolamellar body recrystallization and thylakoid formation in barley etioplasts. J Cell Sci 8: 185–200
Bruce BD (1998) The role of lipids in plastid protein transport. Plant Mol Biol 38: 223–246
Charvolin J, Sadoc J-F (1996) Ordered bicontinuous films of amphiphiles and biological membranes. Philos Trans R Soc Lond A 354: 2173–2192
Cooke RJ, Saunders PF, Kendrick RE (1975) Red light induced production of gibberellin-like substances in homogenates of etiolated wheat leaves and in suspensions of intact etioplasts. Planta 124: 319–328
Gunning BES (1965) The greening process in plastids 1: the structure of the prolamellar body. Protoplasma 60: 111–130
—, Jagoe MP (1967) The prolamellar body. In: Goodwin TW (ed) Biochemistry of chloroplasts, vol 2. Academic Press, London, pp 655–676
—, Steer MW (1975) Ultrastructure and the biology of plant cells. Edward Arnold, London
— — (1996) Plant cell biology: structure and function. Jones and Bartlett, Boston
Henningsen KW, Boynton JE (1969) Macromolecular physiology of plastids VII: the effect of a brief illumination on plastids of dark-grown barley leaves. J Cell Sci 5: 757–793
— — (1970) Macromolecular physiology of plastids VIII: pigment and membrane formation in plastids of barley greening under low light intensity. J Cell Biol 44: 290–304
Hyde S, Andersson S, Larsson K, Blum Z, Landh T, Lidin S, Ninham BW (1997) The language of shape: the role of curvature in condensed matter: physics, chemistry and biology. Elsevier, Amsterdam
Ikeda T (1968) Analytical studies on the structure of prolamellar body. Bot Mag Tokyo 81: 517–527
Israelachvili J, Wolfe J (1980) The membrane geometry of the prolamellar body. Protoplasma 100: 315–321
Jeffrey GA (1984) Hydrate inclusion compounds. In: Atwood JL, Davies JED, MacNicol DD (eds) Inclusion compounds vol 1: structural aspects of inclusion compounds formed by inorganic and organometallic host lattices. Academic Press, London, pp 135–190
Larsson K, Fonteil K, Krog N (1980) Structural relationships between lamellar, cubic and hexagonal phases in monolyceride water systems: possibility of cubic structures in biological systems. Chem Phys Lipids 27: 321–328
Lütz C (1986) Prolamellar bodies. In: Staehelin LA, Arntzen CJ (eds) Photosynthesis III: photosynthetic membranes and light harvesting systems. Springer, Berlin Heidelberg New York Tokyo, pp 683–692 (Pirson A, Zimmermann MH [eds] Encylopedia of plant physiology, new series, vol 19)
Luzzati V (1997) Biological significance of lipid polymorphism: the cubic phases. Curr Opin Struct Biol 7: 661–668
—, Vargas R, Mariani P, Gulik A, Delacroix H (1993) Cubic phases of lipid-containing systems: elements of a theory and biological connotations. J Mol Biol 229: 540–551
Murakami S, Yamada N, Nagano M, Osumi M (1985) Three-dimensional structure of the prolamellar body in squash etioplasts. Protoplasma 128: 147–156
Murphy DJ (1986) Structural properties and molecular organization of the acyl lipids of photosynthetic membranes. In: Staehelin LA, Arntzen CJ (eds) Photosynthesis III: photosynthetic membranes and light harvesting systems. Springer, Berlin Heidelberg New York Tokyo, pp 713–725 (Pirson A, Zimmermann MH [eds] Encyclopedia of plant physiology, new series, vol 19)
O'Keeffe M (1999) Crystal structures as periodic foams and vice versa. In: Sadoc JF, Rivier N (eds) Foams and emulsions. Kluwer, Dordrecht, pp 403–422
Osumi M, Yamada N, Nagano M, Murakami S, Baba N, Oho E, Kanaya K (1984) Three-dimensional observation of the prolamellar bodies in etioplasts of squashCucurbita moschata. Scanning Electron Microsc 1: 111–119
Panke D (1968) Polyhedral clathrate hydrates XV: the structure of 4(CH3)3N · 41H2O. J Chem Phys 48: 2990–2996
Reinbothe C, Lebedev N, Reinbothe S (1999) A protochlorophyllide light-harvesting complex involved in de-etiolation of higher plants. Nature 397: 80–84
Rosinski J, Rosen WG (1972) Chloroplast development: fine structure and chlorophyll synthesis. Q Rev Biol 47: 160–191
Sperling U, Franck F, van Cleve B, Frick G, Apel K, Armstrong GA (1998) Etioplast differentiation inArabidopsis: both PORA and PORB restore the prolamellar body and photoactive protochlorophyllide-F655 to thecopl photomorphogenic mutant. Plant Cell 10: 283–296
Sprague SG, Staehelin LA (1984) Effects of reconstitution method on the structural organization of isolated chloroplast membrane lipids. Biochim Biophys Acta 777: 306–322
Staehelin LA (1986) Chloroplast structure and supramolecular organisation of photosynthetic membranes. In: Staehelin LA, Arntzen CJ (eds) Photosynthesis III: photosynthetic membranes and light harvesting systems. Springer, Berlin Heidelberg New York Tokyo, pp 1–84 (Pirson A, Zimmermann [eds] Encyclopedia of plant physiology, new series, vol 19)
Sundqvist C, Dahlin C (1997) With chlorophyll pigments from prolamellar bodies to light-harvesting complexes. Physiol Plant 100: 748–759
von Wettstein D, Gough S, Kannangara CG (1995) Chlorophyll biosynthesis. Plant Cell 7: 1039–1057
Walles B, Hudak J (1975) A comparative study of chloroplast morphogenesis in seedlings of some conifers (Larix decidua, Pinus sylvestris andPicea abies). Stud For Suec 127: 5–22
Weaire D, Phelan, R (1996) Cellular structures in three dimensions. Philos Trans R Soc Lond A 354: 1989–1997
Wehrmeyer W (1965a) Zur Kristallgitterstruktur der sogenannten Prolamellarkörper in Proplastiden etiolierter Bohnen I: Pentagondodekaeder als Mittelpunkt konzentrischer Prolamellarkörper. Z Naturforsch 20b: 1270–1278
— (1965b) Zur Kristallgitterstruktur der sogenannten Prolamel-larkörper in Proplastiden etiolierter Bohnen II: Zinkblendegitter als Muster tubulärer Anordnungen in Prolamellarkörpern. Z Naturforsch 20b: 1278–1288
— (1965c) Zur Kristallgitterstruktur der sogenannten Prolamellarkörper in Proplastiden etiolierter Bohnen III: Wurtzitgitter als Muster tubulärer Anordnungen in Prolamellarkörpern. Z Naturforsch 20b: 1288–1296
— (1967) Prolamellar bodies: structure and development. In: Croisance et vieillissement des chloroplastes. Masson et Cie, Paris, pp 62–67
Weier TE, Brown DL (1970) Formation of the prolamellar body in 8-day, dark-grown seedlings. Am J Bot 57: 267–275
—, Stocking CR, Shumway LK (1966) The photosynthetic apparatus of higher plants. Brookhaven Symp Biol 19: 353–374
Wellburn AR (1987) Plastids. Int Rev Cytol Suppl 17: 149–210
Wellburn FAM, Wellburn AR (1971) Developmental changes occurring in isolated intact etioplasts. J Cell Sci 9: 271–287
Williams R (1979) The geometrical foundation of natural structure: a source book of design. Dover, New York
Williams WP, Selstam E, Brain T (1998) X-ray diffraction studies of the structural organisation of prolamellar bodies isolated fromZea mays. FEBS Lett 422: 252–254
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunning, B.E.S. Membrane geometry of “open” prolamellar bodies. Protoplasma 215, 4–15 (2001). https://doi.org/10.1007/BF01280299
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01280299