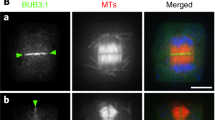
Summary
The distribution of microtubules and actin microfilaments during caffeine-induced inhibition of cell plate formation has been studied in livingTradescantia stamen hair cells. Previous studies have shown that caffeine allows cell plate initiation but prevents its completion, resulting in binucleate cells. In the present study, confocal microscopy of cells microinjected with fluorescent brain tubulin or phalloidin, and cultured in the presence 5 mM caffeine, revealed that the initiation and early lateral expansion phase of the phragmoplast occur normally. However, caffeine completely inhibits the formation of the cytoskeletal torus which occurs in untreated cells during the late stages of cell plate and phragmoplast expansion. Caffeine further causes the disintegration of the incomplete cell plate. The results allow us to distinguish two phases in cell plate and phragmoplast growth: the initiation and early expansion phase, which is not affected by caffeine, and the late lateral expansion phase, which is completely inhibited in the presence of caffeine. Also in this study, the use of a high phalloidin concentration has revealed structural detail about the actin microfilaments involved in cell plate formation: microfilaments are observed that link the expanding edge of the phragmoplast with the cortical division site. In addition, cortical actin patches are observed within the actin depleted zone that might play a role in guidance of phragmoplast and cell plate expansion.
Similar content being viewed by others
References
Asada T, Shibaoka H (1994) Isolation of polypeptides with microtubule-translocating activity from phragmoplasts of tobacco BY-2 cells. J Cell Sci 107: 2249–2257
Assmann SM (1995) Cyclic AMP as a second messenger in higher plants (status and future prospects). Plant Physiol 108: 885–889
Baoge Z, Aigiu G, Xiangdong D, Yuxuan G, Zixian L (1994) Effects of caffeine or EDTA post-treatment on EMS mutagenesis in soybean. Mutat Res 334: 157–159
Baskin TI, Cande WZ (1990) The structure and function of the mitotic spindle in flowering plants. Annu Rev Plant Physiol Plant Mol Biol 41: 277–315
Bassani JWM, Bassani RA, Bers DM (1993) Twitch-dependent SR Ca accumulation and release in rabbit ventricular myocytes. Am J Physiol 265: C533-C540
Battey NH, Blackbourn HD (1993) The control of exocytosis in plant cells. New Phytol 125: 307–338
Becerra J, López-Sáez JF (1978) Effects of caffeine, calcium and magnesium on plant cytokinesis. Exp Cell Res 111: 301–308
Bonsignore CL, Hepler PK (1985) Caffeine inhibition of cytokinesis: dynamics of cell plate formation-deformation in vivo. Protoplasma 129: 28–35
Buchter RW, Sutherland EW (1962) Adenosine-3′,5′-phosphate in biological materials. 1. Purification and properties of 3′,5′-nucleotide phospodiesterase and the use of this enzyme to characterize adenosine 3′,5′ phosphate in human urine. J Biol Chem 237: 1244–1250
Chen TL, Wolniak SM (1989) Lithium induces cell plate dispersion during cytokinesis inTradescantia. Protoplasma 141: 56–63
Clayton L, Lloyd CW (1985) Actin organization during the cell cycle in meristematic plant cells. Exp Cell Res 156: 213–238
Cleary AL, Gunning BES, Wasteneys GO, Hepler PK (1992) Microtubule and F-actin dynamics at the division site in livingTradescantia stamen hair cells. J Cell Sci 103: 977–988
Galione A, White A (1994) Ca2+ release induced by cyclic ADP-ribose. Trends Cell Biol 4: 431–436
Gillaspy GE, Keddie JS, Oda K, Gruissem W (1995) Plant inositol monophosphatase is a lithium-sensitive enzyme encoded by a multigene family. Plant Cell 7: 2175–2185
Gunning BES (1982) The cytokinetic apparatus: its development and spatial regulation. In: Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 229–292
—, Wick SM (1985) Preprophase bands, phragmoplasts, and spatial control of cytokinesis. J Cell Sci Suppl 2: 157–179
Hardham AR, McCully ME (1982) Reprogramming of cells following wounding in pea roots. II. The effects of caffeine and colchicine on the development of new vascular elements. Protoplasma 112: 152–166
Hepler PK (1982) Endoplasmic reticulum in the formation of the cell plate and plasmodesmata. Protoplasma 111: 121–133
—, Bonsignore CL (1990) Caffeine inhibition of cytokinesis: ultrastructure of cell plate formation/degradation. Protoplasma 157: 182–192
Hush JM, Wadsworth P, Callaham DA, Hepler PK (1994) Quantification of microtubule dynamics in living plant cells using fluorescence redistribution after photobleaching. J Cell Sci 107: 775–784
Jones MGK, Payne HL (1977) Cytokinesis inImpatiens balsamina and the effect of caffeine. Cytobios 20: 79–91
Jürgens M, Hepler LH, Rivers BA, Hepler PK (1994) BAPTA-calcium buffers modulate cell plate formation in stamen hairs ofTradescantia: evidence for calcium gradients. Protoplasma 183: 86–99
Kakimoto T, Shibaoka H (1987) Actin filaments and microtubules in the preprophase band and phragmoplast of tobacco cells. Protoplasma 140: 151–156
— —, (1988) Cytoskeletal ultrastructure of phragmoplast-nuclei complexes isolated from cultured tobacco cells. Protoplasma Suppl 2: 95–103
Kauss H (1987) Some aspects of calcium-dependent regulation in plant metabolism. Annu Rev Plant Physiol 38: 47–72
Keifer AQ, Callaham DA, Hepler PK (1992) Inhibitors of cell division and protoplasmic streaming fail to cause a detectable effect on intracellular calcium levels in stamen hair cells ofTradescantia virginiana L. Planta 186: 361–366
Lloyd CW, Traas JA (1988) The role of F-actin in determining the division plane of carrot suspension cells. Drug studies. Development 102: 211–222
Mineyuki Y, Gunning BES (1990) A role for preprophase bands of microtubules in maturation of new cell walls, and a general proposal on the function of preprophase band sites in cell division in higher plants. J Cell Sci 97: 527–537
—, Letham DS, Hocart C (1989) New 3-substituted xanthines: potent inhibitors of cell plate formation. Cell Biol Int Rep 13: 129–136
Palevitz BA (1987) Accumulation of F-actin during cytokinesis inAllium. Correlation with microtubule distribution and the effects of drugs. Protoplasma 141: 24–32
Pierson ES, Miller DD, Callaham DA, van Aken J, Hackett G, Hepler PK (1996) Tip-localized calcium entry fluctuates during polen tube growth. Dev Biol 174: 160–173
Samuels AL, Staehelin LA (1996) Caffeine inhibits cell plate formation by disrupting membrane reorganization just after the vesicle fusion step. Protoplasma 195: 144–155
—, Giddings TH Jr, Staehelin LA (1995) Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol 130: 1345–1357
Schmit AC, Lambert AM (1990) Microinjected fluorescent phalloidin in vivo reveals the F-actin dynamics and assembly in higher plant mitotic cells. Plant Cell 2: 129–138
Schopfer CR, Hepler PK (1991) Distribution of membranes and the cytoskeleton during cell plate formation in pollen mother cells ofTradescantia. J Cell Sci 100: 717–728
Seagull RW, Falconer MM, Weerdenburg CA (1987) Microfilaments: dynamic arrays in higher plant cells. J Cell Biol 104: 995–1004
Subbaiah CC, Bush DS, Sachs MM (1994) Elevation of cytosolic calcium proceeds anoxic gene expression in maize suspensioncultured cells. Plant Cell 6: 1747–1762
Tanaka Y, Tashjian AH (1993) Thimerosal potentiates Ca2+ release mediated by both the inositol 1,4,5-triphosphate and the ryanodine receptors in sea urchin eggs. J Biol Chem 269: 11247–11253
Tretyn A, Czaplewska J, Cymerski M, Kopcewicz J, Kendrick RE (1994) The mechanism of calcium action on flowering induction inPharbitis nil. J Plant Physiol 144: 562–568
Vites A, Pappano AJ (1992) Ruthenium red selectivity presents Ins (1,4,5)P3 — but not caffeine-gated calcium release in avian atrium. Am J Physiol 262: H268-H277
Weber A (1968) The mechanism of the action of caffeine on sarcoplasmic reticulum J Gen Physiol 52: 760–772
Wick SM (1991) The preprophase band. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 231–244
Wolniak SM, Bart KM (1985) The buffering of calcium with quin2 reversibly forestalls anaphase onset in stamen hair cells ofTradescantia. Eur J Cell Biol 39: 33–40
Zhang D, Wadsworth P, Hepler PK (1990) Microtubule dynamics in living dividing plant cells: confocal imaging of microinjected fluorescent brain tubulin. Proc Natl Acad Sci USA 87: 8820–8824
— — — (1993) Dynamics of microfilaments are similar but distinct from microtubules during cytokinesis in living, dividing plant cells. Cell Motil Cytoskeleton 24: 151–155
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Valster, A.H., Hepler, P.K. Caffeine inhibition of cytokinesis: effect on the phragmoplast cytoskeleton in livingTradescantia stamen hair cells. Protoplasma 196, 155–166 (1997). https://doi.org/10.1007/BF01279564
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01279564