Summary
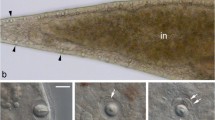
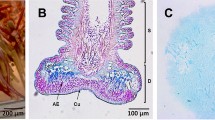
The heliozoonActinophrys sol is characterized by needle-like axopodia radiating from the spherical cell body. When helio-zoons capture food organisms, the prey is caught by adhesion to the surface of axopodia where numerous extrusomes are present close to the plasma membrane. To understand the molecular mechanism by which the heliozoons capture prey organisms, crude isolation and characterization of the adhesive substance was carried out. Prey flagellates (Chlorogonium elongatum) adhered and aggregated to remnants of heliozoon cells which had been killed by freezing or treatment at high temperature (80 °C for 10 min). Isolated extrusomes, which were prepared as the supernatant of cells homogenized and centrifuged after freezing and thawing, showed strong adhesion to the prey flagellates which responded to the supernatant by adhering their flagella and cell bodies to each other to form bouquet-like cell clusters. The adhesive substance was further extracted from heat-treatedA. sol. This fraction contained filamentous material similar to the secreted contents of the extrusomes observed during feeding. Its adhesive activity was not inhibited by trypsin treatment.
Similar content being viewed by others
References
Grebecki A, Hausmann K (1992) Surface coat shedding and axopodial movements inActinophrys sol. Eur J Protistol 28: 390–397
— — (1993) Motor behaviour of prey during first steps of food capture byActinophrys sol. Acta Protozool 32: 157–164
Harumoto T (1994) The role of trichocyst discharge and backward swimming in escaping behavior ofParamecium fromDileptus margaritifer. J Eukaryot Microbiol 41: 560–564
—, Miyake A (1991) Defensive function of trichocysts inParamecium. J Exp Zool 260: 84–92
Hausmann K (1978) Extrusive organelles in protists. Int Rev Cytol 52: 197–276
—, Hülsmann N (1996) Protozoology, 2nd edn. Georg Thieme, Stuttgart
—, Patterson DJ (1982) Pseudopod formation and membrane production during prey capture by a heliozoon (Feeding byActinophrys, II). Cell Motil 2: 9–24
Knoll G, Haacke-Bell B, Plattner H (1991) Local trichocyst exocytosis provides an efficient escape mechanism forParamecium cells. Eur J Protistol 27: 381–385
Kugrens P, Lee RE, Corliss JO (1994) Ultrastructure, biogenesis, and functions of extrusive organelles in selected non-ciliate protists. Protoplasma 181: 164–190
Linnenbach M, Hausmann K, Patterson DJ (1983) Ultrastructural studies on the food vacuole cycle of a heliozoon (Feeding byActinophrys, III). Protoplasma 115: 43–51
Miyake A, Haramoto T (1996) Defensive function of trichocysts inParamecium against the predatory ciliateMonodinium balbiani. Eur J Protistol 32: 128–133
— —, Salvi B, Rivola V (1990) Defensive function of pigment granules inBlepharisma japonicum. Eur J Protistol 25: 310–315
Patterson DJ, Hausmann K (1981) Feeding byActinophrys sol (Protista, Heliozoa) 1: light microscopy. Microbios 31: 39–55
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17: 208–212
Sakaguchi M, Hausmann K (1997) Factors involved in food capture byActinophryrs sol. In: Abstracts of the 10th International Congress of Protozoology, Sydney, New South Wales, 21–25 July, 1997, p 173
Shigenaka Y, Maruoka T, Toyohara A, Suzaki T (1979) Studies on cell fusion of heliozoons V: cell membrane fluidity and surface coat in close relation to the fusion reaction. Annot Zool Japon 52: 163–178
—, Yano K, Yogosawa R, Suzaki T (1982) Rapid contraction of the microtubule-containing axopodia in a large heliozoonEchinosphaerium. In: Sakai H, Mohri H, Borisy GG (eds) Biological functions of microtubules and related structures. Academic Press, New York, pp 105–114
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26: 31–43
Suzaki T, Shigenaka Y, Watanabe S, Toyohara A (1980) Food capture and ingestion in the large heliozoonEchinosphaerium nucleofilum. J Cell Sci 42: 61–79
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakaguchi, M., Hausmann, K. & Suzaki, T. Food capture and adhesion by the heliozoonActinophrys sol . Protoplasma 203, 130–137 (1998). https://doi.org/10.1007/BF01279469
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01279469