Summary
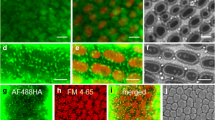
This paper describes the uptake of Lucifer Yellow carbohydrazide and fluorescent dextrans labeled with fluorescein isothiocyanate or Sodium Green (molecular masses ranging from 522 to 2 × 106 Da) byDunaliella spp. halotolerant unicellular green algae isolated from salt pools in the Sinai peninsula. The fluorescent dyes were taken up into a set of vesicles around the nucleus and just above the chloroplast. It proved impossible to inhibit uptake of the fluorescent compounds in cells treated with a large variety of metabolic and other inhibitors. Cell labeling was complete within half a minute of addition of fluorescent compounds to the outside medium; efflux was equally rapid. The results are interpreted in terms of an endocytotic process whereby the outside medium, together with any substance dissolved in it, remains within vesicles enclosed within the cell body but cycles rapidly between the plasma membrane and the interior of the cell. The outside medium does not pass across the vesicular membrane, nor enters the cytosol.
Similar content being viewed by others
Abbreviations
- LYCH:
-
Lucifer Yellow carbohydrazide
- FITC:
-
fluorescein-5-isothiocyanate
- TCA:
-
trichloroacetic acid
- DMSO:
-
dimethylsulfoxide
- NEM:
-
N-ethyl maleimide
- DNP:
-
dinitrophenol
- CCCP:
-
m-chlorocarbonyl-cyanide phenylhydrazone
- APM:
-
amiprophos-methyl
References
Beech PL, Wetherbee R (1988) Observations on the flagellar apparatus and peripheral endoplasmic reticulum of the coccol ithophoridPleurochrysis carterae. Phycologia 27: 142–158
Ben-Amotz A (1974) Osmoregulation mechanism in the halophylic algaDunaliella parva. In: Zimmermann U, Dainty J (eds) Membrane transport in plants. Springer, Berlin Heidelberg New York, pp 95–100
—, Avron M (1973) The role of glycerol in the osmotic regulation of the halophilic algaDunaliella parva. Plant Physiol 51: 875–878
—, Ginzburg BZ (1969) Light-induced proton uptake in whole cells ofDunaliella parva. Biochim Biophys Acta 183: 144–154
Bental M, Degani H, Avron M (1988) 23Na-NMR studies of the intracellular sodium ion concentration in the halotolerant algaDunaliella salina. Plant Physiol 87: 813–817
Betz WJ, Angleson JK (1998) The synaptic vesicle cycle. Annu Rev Physiol 60: 347–363
Bock RM, Ling N-S, Morell SA, Lipton SH (1956) Ultraviolet absorption spectra of adenosine-5′-triphosphate and related 5′-ribonucleotides. Arch Biochem Biophys 62: 253–264
Borowitzka LJ, Brown AD (1974) The salt relations of marine and halophylic species of the unicellular green algaDunaliella. Arch Microbiol 96: 37–52
Bowers B, Olszewski TE (1972) Pinocytosis inAcanthamoeba: kinetics and morphology. J Cell Biol 53: 681–694
Clayton MN, Ashburner CM (1994) Secretion of phenolic bodies following fertilization inDurvillaea potatorum (Durvillaeales, Phaeophyta). Eur J Phycol 29: 1–9
Coleman J, Evans D, Hawes C, Horsley D, Cole L (1987) Structure and molecular organization of higher plant coated vesicles. J Cell Sei 88: 35–45
Craigie JS, McLachlan J (1964) Glycerol as a photosynthetic product inDunaliella tertiolecta Butcher. Can J Bot 42: 777–778
Cunningham RF, Israili ZH, Dayton PG (1981) Clinical pharmacokinetics of probenecid. Clin Pharmacol Kinet 6: 135–151
de Duve C (1963) “Endocytosis”, a note. In: de Reuck AVS, Cameron MP (eds) Lysosomes. Little, Brown and Co, Boston, p 126
Diekmann W, Hedrich R, Raschke K, Robinson DG (1993) Osmocytosis and vacuolar fragmentation in guard cell protoplasts: their relevance to osmotically-induced volume changes in guard cells. J Exp Bot 44: 1569–1577
Domozych DS, Nimmons TT (1992) The contractile vacuole as an endocytic organelle of the chlamydomonad flagellateGloeomonas kupferi (Volvocales, Chlorophyta). J Phycol 28: 809–816
Ehrenfeld J, Cousin J-L (1982) Ionic regulation of the unicellular green algaDunaliella tertiolecta. J Membr Biol 70: 47–57
— — (1984) Ionic regulation of the unicellular green algaDunaliella tertiolecta: response to hypertonic shock. J Membr Biol 77: 45–55
Emons, AMC, Traas JA (1986) Coated pits and coated vesicles on the plasma membrane of plant cells. Eur J Cell Biol 41: 57–64
Fisher M, Gokhman I, Pick U, Zamir A (1997) A structurally novel transferrin-like protein accumulates in the plasma membrane of the unicellular green algaDunaliella salina grown in high salinities. J Biol Chem 272: 1565–1570
Fowke LC, Tanchak MA (1988) The structure and function of plant coated vesicles. In: Pais MSS, Mavituna F, Novais JM (eds) Plant cell biotechnology. Springer, Berlin Heidelberg New York Tokyo, pp 153–163 (NATO ASI Series, series H, vol 18)
— —, Galway ME (1991) Ultrastructural cytology of the endocytotic pathway in plants. In: Hawes CR, Coleman JOD, Evans DE (eds) Endocytosis, exocytosis and vesicle traffic in plants. Cambridge University Press, Cambridge, pp 15–40 (Society for Experimental Biology Seminar series, vol 45)
Ginzburg BZ (1978) Regulation of cell volume and osmotic pressure inDunaliella. In: Caplan SR, Ginzburg M (eds) Energetics and structure of halophilic microorganisms. Elsevier, Amsterdam, pp 543–558
Ginzburg M (1969) The unusual permeability of two halophilic unicellular organisms. Biochim Biophys Acta 173: 370–5/6
— (1987)Dunaliella: a green alga adapted to salt. Adv Bot Res 14: 93–183
—, Richman L (1985) Permeability of wholeDunaliella cells to glucose. J Exp Bot 36: 1959–1968
—, Ginzburg BZ, Wayne R (1997) The plasma membrane ofDunaliella is more than a plasma membrane! Plant Physiol 114: S42, abstract 120
Hopkins CR, Gibson A, Shipman M, Miller K (1990) Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature 346: 335–338
Karni L, Avron M (1988) Ion content of the halotolerant algaDunaliella salina. Plant Cell Physiol 29: 1311–1314
Lenhard JM, Mayorga L, Stahel PD (1992) Characterisation of endosome-endosome fusion in a cell free system usingDictyostelium discoideum. J Biol Chem 267: 1896–1903
Low PS, Chandra S (1994) Endocytosis in plants. Annu Rev Plant Physiol Plant Mol Biol 45: 609–631
Maeda M, Thompson GA (1986) On the mechanism of rapid plasma membrane and chloroplast envelope expansion inDunaliella salina exposed to hyperosmotic shock. J Cell Biol 102: 289–297
Marsh M, Helenius A (1980) Adsorptive endocytosis of semliki forest virus. J Mol Biol 142: 439–454
McFadden GI, Preisig HR, Melkonian M (1986) Golgi apparatus activity and membrane flow during scale biogenesis in the green flagellateScherffelia dubia (Prasinophyceae) II: cell wall secretion and assembly. Protoplasma 131: 174–184
Miller DM, de Ruijter CA, Emons AMC (1997) From signal to form: aspects of the cytoskeleton-plasma membrane-cell wall continuum in root hair tips. J Exp Bot 48: 1881–1896
Miller TM, Heuser JE (1984) Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J Cell Biol 98: 685–698
Mukherjee S, Ghosh RN, Maxfield FR (1997) Endocytosis. Physiol Rev 77: 760–803
O'Driscoll D, Hann C, Read SM, Steer MW (1993) Endocytotic uptake of fluorescent dextrans by pollen tubes grown in vitro. Protoplasma 175: 126–130
O'Neil RM, La Claire JW II (1988) Endocytosis and membrane dynamics during the wound response of the green algaBoergenesia. Cytobios 53: 113–125
Oliviera L, Bisalputra T, Antia NJ (1980) Ultrastructural observation of the surface coat ofDunaliella tertiolecta from staining with cationic dyes and enzyme treatments. New Phytol 85: 385–392
Oparka KJ, Prior DAM, Harris N (1990) Osmotic induction of fluidphase endocytosis in onion epidermal cells. Planta 180: 555–561
—, Murant EA, Wright KM, Prior DAM, Harris N (1991) The drug probenecid inhibits the vacuolar accumulation of fluorescent anions in onion epidermal cells. J Cell Sci 99: 557–563
Patzak A, Annis D, Langley K (1987) Membrane recycling after exocytosis: an ultrastructure study of cultured chromaffin cells. Exp Cell Res 171: 346–356
Picton JM, Steer MW (1983) Membrane recycling and the control of secretory activity in pollen tubes. J Cell Sci 63: 303–310
Pick U, Karni L, Avron M (1986) Determination of ion content and ion fluxes in the halotolerant algaDunaliella salina. Plant Physiol 81: 92–96
Prescianotto-Baschong C, Riezman H (1998) Morphology of the yeast endocytotic pathway. Mol Biol Cell 9: 173–189
Roszak R, Rambour S (1997) Uptake of Lucifer Yellow by plant cells in the presence of endocytotic inhibitors. Protoplasma 199: 198–207
Samuels AL, Bisapultra T (1990) Endocytosis in elongating root cells ofLobelia erinus. J Cell Sci 97: 157–165
Skulachev VP (1994) The latest news from the sodium world. Biochim Biophys Acta 1187: 216–221
Steinman RM, Brodie SE, Cohn ZA (1983) Membrane flow during pinocytosis: a stereologic analysis. J Cell Biol 68: 665–687
Tanchak M, Griffing L, Mersey B, Fowke L (1984) Endocytosis of cationized ferritin by coated vesicles of soybean protoplasts. Planta 162: 481–486
—, Rennie PJ, Fowke LC (1988) Ultrastructure of the partially coated reticulum and dictyosomes during endocytosis by soybeans protoplasts. Planta 175: 433–441
Thilo L (1985) Selective internalization of granule membrane after secretion in mast cells. Proc Natl Acad Sci USA 82: 1711–1715
Thomas P, Lee A, Wong J, Almers W (1994) A triggered mechanism retrieves membrane in seconds after Ca2+-stimulated exocytosis in single pituitary cells. J Cell Biol 124: 667–675
Trezzi F, Galli MG, Bellini E (1965) L'osmoresistenza diDunaliella salina: richerche ultrastrutturali. G Bot Ital 72: 255–263
van Deurs B, Petersen O, Olsnes S, Sandvig K (1989) The ways of endocytosis. Int Rev Cytol 117: 131–177
von Grafenstein H, Roberts CS, Baker PF (1986) Kinetic analysis of the triggered exocytosis/endocytosis secretory cycle in cultured bovine adrenal medullary cells. J Cell Biol 103: 2343–2352
Watts C, Marsh M (1992) Endocytosis: what goes in and how? J Cell Sci 103: 1–8
Wayne R, Kadota A, Watanabe M, Fumya M (1991) Photomovement inDunaliella salina fluence rate: response curves and action spectra. Planta 184: 515–524
Zhang XQ, Dubacq J-P, Alfsen A (1993) Biochemical and cytological evidence for the stimulation of clathrin-coated pit (vesicle) formation by exogenous folic acid inDunaliella salina (Chlorophyta). J Phycol 29: 302–209
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ginzburg, M., Ginzburg, B.Z. & Wayne, R. Ultrarapid endocytotic uptake of large molecules inDunaliella species. Protoplasma 206, 73–86 (1999). https://doi.org/10.1007/BF01279254
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01279254