Summary
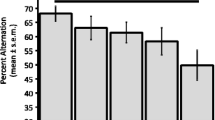
Thein vivomicrodialysis technique was used to measure extracellular concentrations of acetylcholine (ACh) in the neostriatum (NS) and nucleus accumbens (NAc) of freely moving rats after intraperitoneal administration of the muscarinic receptor antagonist scopolamine (0.5mg/kg) or vehicle. Simultaneously, behavior was monitored. The administration of scopolamine induced an increase in extracellular ACh levels in the NS, which reached a maximum of about 185% within one hour after injection and returned to baseline values about three hours after injection. In the NAc, an increase of similar time-course was observed; however, this increase reached a maximum of 250%, which was significantly higher than the one observed in NS. These changes in ACh levels were accompanied by enhanced locomotion, rearing and grooming; however, the behavioral changes were of shorter time-course than those of extracellular ACh. The injection of vehicle did not affect ACh levels in NS, but induced a significant increase (60%) in the NAc. The levels of behavioral activity after vehicle injection did not differ from pre-injection levels. These results suggest, that the cholinergic systems in the NAc and NS are differently affected by peripheral administration of both scopolamine and vehicle. The differential effects of scopolamine in NS and NAc could reflect pharmacodynamic differences between these two striatal brain areas, perhaps due to a higher density of cholinergic interneurons or muscarinic autoreceptors in the NAc in comparsion to the NS. However, the increase of extracellular ACh observed after vehicle injection suggests that factors such as aversive stimulation through the injection procedure can increase ACh release in the NAc and that such a mechanism can interact within the action of scopolamine. Thus, the stronger action of scopolamine on extracellular ACh in the NAc might be an additive effect of the drug with that of the injection procedure.
Similar content being viewed by others
References
Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ (1989) Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem 52: 1655–1658
Amaral DG, Price JL, Pitkänen A, Carmichael ST (1992) Anatomical organization of the primate amygdaloid complex. In: Aggelton JP (eds) The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss, New York, pp 1–66
Bernard V, Normand E, Bloch B (1992) Phenotypical characterization of rat striatal neurons expressing muscarinic receptor genes. J Neurosci 12: 3591–3600
Blaha CD, Lane RF (1987) Chronic treatment with classical and atypical antipsychotic drugs differentially decreases dopamine release in striatum and nucleus accumbens in vivo. Neurosci Lett 78: 199–204
Boix F, Huston JP, Schwarting RKW (1992a) The C-terminal fragment of substance P enhances dopamine release in nucleus accumbens but not in neostriatum in freely moving rats. Brain Res 592: 181–186
Boix F, Mattioli R, Adams F, Huston JP, Schwarting RKW (1922b) Effects of substance P on extracellular dopamine in neostriatum and nucleus accumbens. Eur J Pharmacol 216: 103–107
Chesselet M-F (1984) Presynaptic regulation of neurotransmitter release in the brain: facts and hypothesis. Neuroscience 12: 347–375
Consolo S, Ladinsky H, Vinci R, Palazzi E, Wang J-X (1987) Anin vivo pharmacological study on muscarinic receptor subtypes regulating cholinergic neurotransmission in rat striatum. Biochem Pharmacol 36: 3075–3077
Cortés R, Palacios JM (1986) Muscarinic cholinergic receptor subtypes in the rat brain. I. Quantitative autoradiographic studies. Brain Res 362: 227–238
Damsma G, Lammerts van Bueren D, Westerink BHC, Horn AS (1987) Determination of acetylcholine and choline in the femtomole range by means of HPLC, a post-column enzyme reactor, and electrochemical detection. Chromatographia 24: 827–831
Damsma G, de Boer P, Westerink BHC, Fibiger HC (1990) Dopaminergic regulation of striatal cholinergic interneurons: an in vivo microdialysis study. Naunyn Schmiedebergs Arch Pharmacol 342: 523–527
Damsma G, Westerink BHC (1991) A microdialysis and automated on-line analysis approach to study central cholinergic transmission in vivo. In: Robinson TE, Justice JB Jr (eds) Microdialysis in the neurosciences. Elsevier, Amsterdam, pp 237–252
Dawson VL, Dawson TM, Wamsley JK (1990) Muscarinic and dopaminergic receptor subtypes on striatal cholinergic interneurons. Brain Res Bull 25: 903–912
Day J, Damsma G, Fibiger HC (1991) Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: an in vivo microdialysis study. Pharmacol Biochem Behav 38: 723–729
de Boer P, Damsma G, Fibiger HC, Timmermann W, de Vries JB, Westerink BHC (1990) Dopaminergic-cholinergic interactions in the striatum: the critical significance of calcium concentrations in brain microdialysis. Naunyn Schmiedebergs Arch Pharmacol 342: 528–534
Di Chiara G, Imperato A (1987) Opposite effects ofmu andkappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther 244: 1067–1080
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278
Florian SM, Kuczenski R, Segal DS (1992) Amphetamine-induced changes in behavior and caudate extracellular acetylcholine. Brain Res 581: 53–58
Goto T, Kuzuya F, Endo H, Tajima T, Ikari H (1990) Some effects of CNS cholinergic neurons on memory. J Neural Transm [Suppl] 30: 1–11
Groenewegen HJ, Berendse HW, Meredith GE, Haber SN, Voorn P, Wolters JG, Lohmann AHM (1991) Functional anatomy of the ventral, limbic system-innervated striatum. In: Willner P, Scheel-Krüger J (eds) The mesolimbic dopamine system: from motivation to action. Wiley, Chichester, pp 19–59
Heimer L, Switzer RD, Van Hoesen GW (1982) Ventral striatum and ventral pallidum. Components of the motor system? TINS 5: 83–87
Huston JP, Hasenöhrl RU, Boix F, Gerhardt P, Schwarting RKW (1993) Sequence-specific effects of neurokinin substance P on memory, reinforcement, and brain dopamine activity. Psychopharmacology 112: 147–162
Ichikawa I, Meltzer HY (1990) Differential effects of repeated treatment with haloperidol and clozapine on dopamine release and metabolism in the striatum and nucleus accumbens. J Pharmacol Exp Ther 256: 348–375
Imperato A, Angelucci L, Casolini P, Zocchi A, Puglisi-Allegra S (1992) Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res 577: 194–199
Karoum F, Egan MF (1992) Dopamine release and metabolism in the rat frontal cortex, nucleus accumbens, and striatum: a comparison of acute clozapine and haloperidol. Br J Pharmacol 105: 703–707
Kása P (1986) The cholinergic system in brain and spinal cord. Prog Neurobiol 26: 211–272
LeDoux JE (1992) Emotion and the amygdala. In: Aggelton JP (ed) The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss, New York, pp 339–351
Le Moal M, Simon H (1991) Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev 771: 155–234
Mash DC, Potter LT (1986) Autoradiographic localisation of M1 and M2 muscarine receptors in the rat brain. Neuroscience 19: 551–564
Meredith GE, Blank B, Groenewegen HJ (1989) The distribution and compartmental organisation of the cholinergic neurons in nucleus accumbens of the rat. Neuroscience 31: 327–345
Meredith GE, Wouterlood FG (1990) Hippocampal and midline thalamic fibers and terminals in relation to the choline acetyltransferase-immunoreactive neurons in nucleus accumbens of the rat: a light and electron microscopic study. J Comp Neurol 296: 204–221
Nakahara D, Ozaki N, Nagatsu T (1993) In vivo microdialysis of neurotransmitters and their metabolites. In: Parves SH, Naoi M, Nagatsu T, Parves S (eds) Methods in neurotransmitter and neuropeptide research. Elsevier, Amsterdam, pp 219–249
Nauta WJH, Domesick VB (1984) Afferent and efferent relationships of the basal ganglia. In: Evered D, O'Connor M (eds) Functions of the basal ganglia. Pitman Publishing, London, pp 3–29 (CIBA Foundation Symposium 107)
Nilsson OG, Kalíen P, Rosengren E, Björklund A (1990) Acetylcholine release in the rat hippocampus as studied by microdialysis is dependent on axonal impulse flow and increases during behavioural activation. Neuroscience 36: 325–338
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, Sydney
Phillips AG, Pfaus JG, Blaha CD (1991) Dopamine and motivated behavior: insights provided byin vivo analyses. In: Willner P, Scheel-Krüger J (eds) The mesolimbic dopamine system: from motivation to action. Wiley, Chichester, pp 199–224
Puglisi-Allegra S, Imperato A, Angelucci L, Cabib S (1991) Acute stress induces time-dependent responses in dopamine mesolimbic system. Brain Res 554: 217–222
Rada P, Pothos E, Mark GP, Hoebel BG (1991) Microdialysis evidence that acetylcholine in the nucleus accumbens is involved in morphine withdrawal and its treatment with clonidine. Brain Res 561: 354–356
Rada PV, Mark GP, Hoebel BG (1993a) In vivo modulation of acetylcholine in the nucleus accumbens of freely moving rats. I. Inhibition by serotonin. Brain Res 619: 98–104
Rada PV, Mark GP, Hoebel BG (1993b) In vivo modulation of acetylcholine in the nucleus accumbens of freely moving rats. II. Inhibition by γy-aminobutyric acid. Brain Res 619: 105–110
Rappaport MS, Sealfon SC, Prikhozhan A, Huntley GW, Morrison JH (1993) Heterogenous distribution of D1, D2 and D5 receptor mRNAs in monkey striatum. Brain Res 616: 242–250
Rougé-Pont F, Piazza PV, Kharouby M, Le Moal M, Simon H (1993) Higher and longer stress-induced increase in dopamine concentrations in the nucleus accumbens of animals predisposed to amphetamine self-administration. A microdialysis study. Brain Res 602: 169–174
Scheel-Krüger J, Willner P (1991) The mesolimbic system: principles of operation. In: Willner P, Scheel-Krüger J (eds) The mesolimbic dopamine system: from motivation to action. Wiley, Chichester, pp 559–597
Schoffelmeer ANM, Van Vliet BJ, Wardeh G, Mulder AH (1986) Muscarinic-receptor mediated modulation of [14C]acetylcholine release from rat neostriatal slices: selective antagonism by gallamine but not pirenzepine. Eur J Pharmacol 128: 219–294
Sharp T, Zetterström T, Ljungberg T, Ungerstedt U (1986) A direct comparison of amphetamine-induced behaviours and regional brain dopamine release in the rat using intracerebral dialysis. Brain Res 401: 322–330
Stoof JC, Drukarch B, de Boer P, Westerink BHC, Groenewegen HJ (1992) Regulation of the activity of striatal cholinergic neurons by dopamine. Neuroscience 47: 755–770
Toide K (1989) Effects of scopolamine on extracellular acetylcholine and choline levels and on spontaneous motor activity in freely moving rats measured by brain dialysis. Pharmacol Biochem Behav 33: 109–113
Toide K, Arima T (1989) Effects of cholinergic drugs on extracellular levels of acetylcholine and choline in rat cortex, hippocampus and striatum studied by brain dialysis. Eur J Pharmacol 173: 133–141
Ungerstedt U (1991) Introduction to intracerebral microdialysis. In: Robinson TE, Justice JB Jr (eds) Microdialysis in the neurosciences. Elsevier, Amsterdam, pp 3–22
Watanabe H, Shimizu H (1989) Effect of anticholinergic drugs on striatal acetylcholine release and motor activity in freely moving rats studied by brain microdialysis. Jpn J Pharmacol 51: 75–82
Watanabe H, Shimizu H, Matsumoto K (1990) Acetylcholine release detected by trans-striatal dialysis in freely moving rats correlates with spontaneous motor activity. Life Sci 47: 829–832
Watson M, Roeske WR, Vickory TW, Smith TL, Akiyama K, Gulya K, Duckles SP, Serra M, Adem A, Norberg A, Gehler DR, Wamsley JK, Yamamura HI (1986) Biochemical and functional basis of putative muscarinic receptor subtypes and its implications. TIPS [Suppl]: 46–55 (Subtype muscarinic receptors)
Woolf NJ (1991) Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 37: 475–524
Yang CR, Mogenson GJ (1984) Electrophysiological responses of neurones in the nucleus accumbens to hippocampal stimulation and the attenuation of the excitatory responses by the mesolimbic dopaminergic system. Brain Res 324: 69–84
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pfister, M., Boix, F., Huston, J.P. et al. Different effects of scopolamine on extracellular acetylcholine levels in neostriatum and nucleus accumbens measured in vivo: possible interaction with aversive stimulation. J. Neural Transmission 97, 13–25 (1994). https://doi.org/10.1007/BF01277959
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01277959