Summary
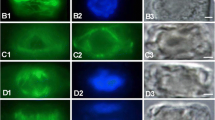
Microtubule reorganization and cell wall deposition have been monitored during the first 30 hours of regeneration of protoplasts of the filamentous green algaMougeotia, using immunofluorescence microscopy to detect microtubules, and the cell-wall stain Tinopal LPW to detect the orientation of cell wall microfibrils. In the cylindrical cells of the alga, cortical microtubules lie in an ordered array, transverse to the long axis of the cells. In newly formed protoplasts, cortical microtubules exhibit some localized order, but within 1 hour microtubules become disordered. However, within 3 to 4 hours, microtubules are reorganized into a highly ordered, symmetrical array centered on two cortical foci. Cell wall synthesis is first detected during early microtubule reorganization. Oriented cell wall microfibrils, co-aligned with the microtubule array, appear subsequent to microtubule reorganization but before cell elongation begins. Most cells elongate in the period between 20 to 30 hours. Elongation is preceded by the aggregation of microtubules into a band intersecting both foci, and transverse to the incipient axis of elongation. The foci subsequently disappear, the microtubule band widens, and microfibrils are deposited in a band which is co-aligned with the band of microtubules. It is proposed that this band of microfibrils restricts lateral expansion of the cells and promotes elongation. Throughout the entire regeneration process inMougeotia, changes in microtubule organization precede and are paralleled by changes in cell wall organization. Protoplast regeneration inMougeotia is therefore a highly ordered process in which the orientation of the rapidly reorganized array of cortical microtubules establishes the future axis of elongation.
Similar content being viewed by others
References
Brown RM, Jr (1985) Cellulose microfibril assembly and orientation: recent developments. J Cell Sci [Suppl] 2: 13–32
Cocking EC (1972) Plant cell protoplasts—isolation and development. Ann Rev Plant Physiol 23: 29–50
Falconer MM, Seagull RW (1985) Xylogenesis in tissue culture: taxol effects on microtubule reorientation and lateral association in differentiating cells. Protoplasma 128: 157–166
— — (1986) Xylogenesis in tissue culture II: microtubules, cell shape and secondary wall patterns. Protoplasma 133: 140–148
Foos K (1970) Mikrotubuli beiMougeotia spec. Z Pflanzenphysiol 62: 201–203
— (1971) Untersuchungen zur Feinstruktur vonMougeotia spec. und zum Bewegungsmechanismus des Chloroplasten. Z Pflanzenphysiol 64: 369–386
Fowke LC, Gamborg OL (1980) Applications of protoplasts to the study of plant cells. Int Rev Cytol 68: 9–51
—,Rennie PJ, Constabel F (1983) Organelles associated with the plasma membrane of tobacco leaf protoplasts. Plant Cell Reports 2: 292–295
Giloh H, Sedat JW (1982) Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science 217: 1252–1255
Green PB, Erickson RO, Richmond PA (1970) On the physical basis of cell morphogenesis. Ann NY Acad Sci 175: 712–731
Hahne G, Hoffmann F (1985) Cortical microtubular lattices: absent from mature mesophyll and necessary for cell division? Planta 166: 309–313
Haigler CH, Benziman M (1982) Biogenesis of cellulose I microfibrils occurs by cell-directed self-assembly inAcetobacter xylinum. In:Brown RM, Jr (ed) Cellulose and other natural polymer systems. Plenum Press, New York, pp 273–297
Heath IB, Seagull RW (1982) Oriented cellulose fibrils and the cytoskeleton: a critical comparison of models. In:Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 163–182
Herth W, Hausser I (1984) Chitin and cellulose fibrillogenesisin vivo and their experimental alteration. In:Dugger WM, Bartnicki-Garcia S (eds) Structure, function, and biosynthesis of plant cell walls. American Society of Plant Physiologists, Rockville, Maryland, pp 89–119
Hotchkiss AT, Jr., Brown RM, Jr. (1984) Microfibril assembly during cell wall regeneration ofMougeotia protoplasts. J Cell Biol 99: 77 a
— — (1985) Complete regeneration ofMougeotia protoplasts. J Phycol 21 [Suppl] 18
Hughes J, McCully ME (1975) The use of an optical brightener in the study of plant structure. Stain Technol 50: 319–329
Itoh T, O'Neil RM, Brown RM, Jr. (1984) Interference of cell wall regeneration ofBoergesenia forbesii protoplasts by Tinopal LPW, a fluorescent brightening agent. Protoplasma 123: 174–183
Kakimoto T, Shibaoka H (1986) Calcium-sensitivity of cortical microtubules in the green algaMougeotia. Plant Cell Physiol 27: 91–101
Lloyd CW, Clayton L, Dawson PJ, Doonan JH, Hulme JS, Roberts IN, Wells B (1985) The cytoskeleton underlying side walls and cross walls in plants: molecules and macromolecular assemblies. J Cell Sci [Suppl] 2: 143–155
—,Slabas AR, Powell AJ, Lowe SB (1980) Microtubules, protoplasts and plant cell shape. Planta 147: 500–506
Maeda H, Ishida N (1967) Specificity of binding of hexapyranosyl polysaccharides with fluorescent brightener. J Biochem 62: 276–278
Marchant HJ (1978 a) Scanning electron microscopy of microtubules associated with the plasma membrane of protoplasts of the algaMougeotia. Scanning Electron Microsc II: 1071–1076
— (1978 b) Microtubules associated with the plasma membrane isolated from protoplasts of the green algaMougeotia. Exp Cell Res 115: 25–30
— (1979) Microtubules, cell wall deposition and the determination of plant cell shape. Nature 278: 167–168
—,Fowke LC (1977) Preparation, culture, and regeneration of protoplasts from filamentous green algae. Can J Bot 55: 3080–3086
—,Hines ER (1979) The role of microtubules and cell-wall deposition in elongation of regenerating protoplasts ofMougeotia. Planta 146: 41–48
Pickett-Heaps JD (1970) Mitosis and autospore formation in the green algaKirchneriella lunaris. Protoplasma 70: 325–347
Pocock MA (1960)Hydrodictyon: a comparative biological survey. J S Afr Bot 26: 167–319
Roberts E, Seagull RW, Haigler CH, Brown RM, Jr (1982) Alteration of cellulose microfibril formation in eukaryotic cells: Calcofluor White interferes with microfibril assembly and orientation inOocystis apiculata. Protoplasma 113: 1–9
Robinson DG, Quader H (1982) The microtubule-microfibril syndrome. In:Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 109–126
Schroeder M, Wehland J, Weber K (1985) Immunofluorescence microscopy of microtubules in plant cells; stabilization by dimethylsulfoxide. Eur J Cell Biol 38: 211–218
Simmonds DH, Setterfield G, Brown DL (1983) Reorganization of microtubules in protoplasts ofVicia hajastana, Grossh. during the first 48 hours of culturing. In:Potrykus I, Harms CT, Hinnen A, Hütter R, King PJ, Shillito RD (eds) Protoplasts 1983 Proc 6th Int Protoplast Symp. Birkhauser Verlag, Basel, pp 212–213
Van der Valk P, Rennie PJ, Connolly JA, Fowke LC (1980) Distribution of cortical microtubules in tobacco protoplasts. An immunofluorescence microscopic and ultrastructural study. Protoplasma 105: 27–43
Vasil IK, Vasil V (1980) Isolation and culture of protoplasts. Int Rev Cytol [Suppl] 11 B: 1–19
Wick SM, Duniec J (1986) Effects of various fixatives on the reactivity of plant cell tubulin and calmodulin in immunofluorescence microscopy. Protoplasma 133: 1–18
Williamson FA, Fowke LC, Weber G, Constabel F, Gamborg O (1977) Microfibril deposition on cultured protoplasts ofVicia hajastana. Protoplasma 91: 213–219
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Galway, M.E., Hardham, A.R. Microtubule reorganization, cell wall synthesis and establishment of the axis of elongation in regenerating protoplasts of the algaMougeotia . Protoplasma 135, 130–143 (1986). https://doi.org/10.1007/BF01277006
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01277006