Summary
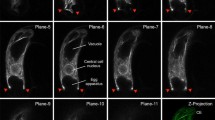
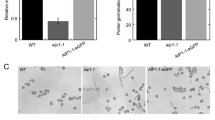
The organization of actin microfilaments (MFs) was studied during pollen development ofBrassica napus cv. Topas. Cells were prepared using three techniques and double labelled for fluorescence microscopy with rhodamine-labelled phalloidin for MFs and Hoechst 33258 for DNA. Microfilaments are present at all stages of pollen development with the exception of tricellular pollen just prior to anthesis. Unicellular microspores contain MFs which radiate from the surface of the nuclear envelope into the cytoplasm. During mitosis MFs form a network partially surrounding the mitotic apparatus and extend into the cytoplasm. Both cytoplasmic and phragmoplast-associated MFs are present during cytokinesis. Nuclear associated-, cytoplasmic, and randomly oriented cortical MFs appear in the vegetative cell of the bicellular microspore. Cortical MFs in the vegetative cell organize into parallel MF bundles (MFBs) aligned transverse to the furrows. The MFBs disappear prior to microspore elongation. At anthesis MFs are restricted to the cortical areas subjacent to the furrows of the vegetative cell. The use of cytochalasin D to disrupt MF function resulted in: (1) displacement of the acentric nucleus in the unicellular microspore; (2) displacement of the spindle apparatus in the mitotic cell; (3) symmetrical growth of the bicellular microspore rather than elongation and (4) inhibition of pollen tube germination in the mature pollen grain. This suggests that MFs play an important role in anchoring the nucleus in the unicellular microspore as well as the spindle apparatus during microspore mitosis, in microspore shape determination and in pollen tube germination.
Similar content being viewed by others
Abbreviations
- MF:
-
microfilament
- MFB:
-
microfilament bundle
- rhph:
-
rhodamine phalloidin
References
Andersland JM, Parthasarathy MV (1993) Conditions affecting depolymerization of actin in plant homogenates. J Cell Sci 104: 1273–1279
Brewbaker JL, Kwack BH (1963) The essential role of calcium ion in pollen germination and pollen tube growth. Amer J Bot 50: 859–865
Brown RC, Lemmon BE (1991 a) Pollen development in orchids 3. A novel generative pole microtubule system predicts unequal pollen mitosis. J Cell Sci 99: 273–281
— — (1991 b) Pollen development in orchids 4. Cytoskeleton and ultrastructure of the unequal pollen mitosis inPhalaenopsis. Protoplasma 167: 183–192
— — (1991 c) Pollen development in orchids 5. A generative cell domain involved in spatial control of the hemispherical cell plate. J Cell Sci 100: 559–565
Burgess J (1970) Microtubules and cell division in the microspores ofDactylorchis fuschii. Protoplasma 69: 253–264
Clayton L, Lloyd CW (1985) Actin organization during the cell cycle in meristematic plant cells. Exp Cell Res 156: 231–238
Cleary AL, Gunning BES, Wasteneys GO, Hepler PK (1992) Microtubule and F-actin dynamics at the division site in livingTradescantia stamen hair cells. J Cell Sci 103: 977–988
Echlin P (1971) The role of the tapetum during microsporogenesis of angiosperms. In: Heslop-Harrison J (ed) Pollen: development and physiology. Butterworths, London, pp 41–61
Fromm M, Callis J, Taylor LP, Walbot V (1987) Electroporation of DNA and RNA into plant protoplasts. Methods Enzymol 153: 351–366
Goodbody KC, Lloyd CW (1990) Actin filaments line up acrossTradescantia epidermal cells, anticipating wound-induced division planes. Protoplasma 157: 92–101
Gunning BES, Wick SM (1985) Preprophase bands, phragmoplasts, and spatial control of cytokinesis. J Cell Sci [Suppl] 2: 157–179
Hause G, Hause B, Van Lammeren AAM (1992) Microtubular and actin filament configurations during microspore and pollen development inBrassica napus cv. Topas. Can J Bot 70: 1369–1376
Hepler PK, Palevitz BA (1974) Microtubules and microfilaments. Annu Rev Plant Physiol 25: 309–362
Helsop-Harrison J (1968) Synchronous pollen mitosis and the formation of the generative cell in massulate orchids. J Cell Sci 3: 457–466
—, Heslop-Harrison Y, Cresti M, Tiezzi A, Ciampolini F (1986) Actin during pollen germination. J Cell Sci 88: 1–8
Hush JM, Overall RL (1992) Re-orientation of cortical F-actin is not necessary for wound-induced microtubule re-orientation and cell polarity establishment. Protoplasma 169: 97–106
Kakimoto J, Shibaoka H (1987) Actin filaments and microtubules in the preprophase band and phragmoplast of tobacco cells. Protoplasma 140: 151–156
Katsuta J, Shibaoka H (1988) The roles of the cytoskeleton and the cell wall in nuclear positioning in tobacco BY-2 cells. Plant Cell Physiol 29: 403–413
Knox B (1984) The pollen grain. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin Heidelberg New York Toyko, pp 197–271
Lancelle SA, Hepler PK (1988) Cytochalasin-induced ultrastructural alterations inNicotiana pollen tubes. Protoplasma [Suppl 2]: 65–75
Lloyd CW (1988) Actin in plants. J Cell Sci 90: 185–188
—, Traas JA (1988) The role of f-actin in determining the division plane of carrot suspension cells. Drug studies. Development 102: 211–221
Mascarenhas J (1975) The biochemistry of angiosperm pollen development. Bot Rev 41: 259–314
McCurdy DW, Gunning BES (1990) Reorganization of cortical actin microfilaments and microtubules at preprophase and mitosis in wheat root-tip cells: a double label immunofluorescence study. Cell Motil Cytoskeleton 15: 76–87
—, Palevitz BA, Gunning BES (1991) Effect of cytochalasins on actin in dividing root tip cells ofAllium andTriticum: a comparative immunocytochemical study. Cell Motil Cytoskeleton 18: 107–112
Murgia M, Detchepare S, Van Went JL, Cresti M (1991)Brassica napus pollen development during generative cell and sperm cell formation. Sex Plant Reprod 4: 176–181
Palevitz BA (1987) Actin in the pre-prophase band ofAllium cepa. J Cell Biol 104: 1515–1519
—, Liu B (1992) Microfilaments (F-actin) in generative cells and sperm: an evaluation. Sex Plant Reprod 5: 89–100
Parthasarathy MV (1985) F-actin architecture in coleoptile epidermal cells. Eur J Cell Biol 39: 1–12
—, Perdue TD, Witztum A, Alvernaz J (1985) Actin network as a normal component of the Cytoskeleton in many vascular plant cells. Amer J Bot 72: 1318–1323
Pierson ES, Derksen J, Traas JA (1986) Organization of microfilaments and microtubules in pollen tubes grown in vitro or in vivo in various angiosperms. Eur J Cell Biol 41: 14–18
Rutten TLM, Derksen J (1990) Organization of actin filaments and outgrowing subprotoplasts from pollen tubes ofNicotiana tabacum L, Planta 180: 471–479
Schmit A-C, Lambert A-M (1987) Characterization and dynamics of cytoplasmic F-actin in higher plant endosperm cells during interphase, mitosis, and cytokinesis. J Cell Biol 105: 2157–2166
— — (1990) Microinjected fluorescent phalloidin in vivo reveals the f-actin dynamics and assembly in higher plant mitotic cells. Plant Cell 2: 129–138
Seagull RW (1990) The effects of microtubule and microfilament disrupting agents on cytoskeletal arrays and wall deposition in developing cotton fibers. Protoplasma 159: 44–59
—, Falconer MM, Weerdenburg CA (1987) Microfilaments: dynamic arrays in higher plant cells. J Cell Biol 104: 995–1004
Simmonds DH (1993) Mechanism of induction of microspore embryogenesis inBrassica napus: significance of the preprophase band of microtubules in the first sporophytic division. In: Akkas N (ed) Biomechanics of active movement and division of cells. Springer, Berlin Heidelberg New York Tokyo, pp 569–574 (NATO ASI series, series H, vol 84)
- Gervais C, Keller WA (1991) Embryogenesis from microspores of embryogenie and non-embryogenic lines ofBrassica napus. In: Rapeseed in a changing world. Proceedings of the Rapeseed Congress, Saskatoon, Saskatchewan, Canada, pp 306–311
Sonobe S, Shibaoka H (1989) Cortical fine actin filaments in higher plant cells visualized by rhodamine-phalloidin after pretreatment with m-maleimidobenzoyl N-hydroxysuccinimide ester. Protoplasma 148: 80–86
Tanaka I, Ito M (1981) Control of division patterns in explanted microspores ofTulipa gesneriana. Protoplasma 108: 329–340
—, Wakabayashi T (1992) Organization of the actin and microtubule Cytoskeleton preceding pollen germination. An analysis using cultured pollen protoplasts ofLilium longiflorum. Planta 186: 473–482
Tang X, Lancelle SA, Hepler PK (1989) Fluorescence microscopic localization of actin in pollen tubes: comparison of actin antibody and phalloidin staining. Cell Motil Cytoskeleton 12: 216–224
Telmer CA, Simmonds DH, Newcomb W (1992) Determination of developmental stage to obtain high frequencies of embryogenie microspores inBrassica napus. Physiol Plant 84: 417–424
—, Newcomb W, Simmonds DH (1993) Microspore development inBrassica napus and the effect of high temperature on division in vivo and in vitro. Protoplasma 172: 154–165
Terasaka O, Niitsu T (1990) Unequal cell division and chromatin differentiation in pollen grain cells. II. Microtubule dynamics associated with the unequal cell division. Bot Mag Toyko 103: 133–142
Tiwari SC, Polito VS (1988) Spatial and temporal organization of actin during hydration, activation, and germination of pollen inPyrus communis L.: a population study. Protoplasma 147: 5–15
— — (1990) Ananalysisoftheroleofactinduringpollenactivation leading to germination in pear (Pyrus communis L.): treatment with cytochalasin D. Sex Plant Reprod 3: 121–129
Traas JA, Doonan JH, Rawlins DJ, Shaw PJ, Watts J, Lloyd CW (1987) An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus. J Cell Sci 105: 387–395
Van Lammeren AAM, Bednara J, Willemse MTM (1989) Organization of the actin Cytoskeleton during pollen development inGasteria verrucosa (Mill.) H. Duval visualized with rhodaminephalloidin. Planta 178: 531–539
Zhang D, Wadsworth P, Hepler PK (1993) Dynamics of microfilaments are similar, but distinct from microtubules during cytokinesis in living, dividing plant cells. Cell Motil Cytoskeleton 24: 151–155
Author information
Authors and Affiliations
Additional information
Dedicated to the memory of Professor John G. Torrey
Rights and permissions
About this article
Cite this article
Gervais, C., Simmonds, D.H. & Newcomb, W. Actin microfilament organization during pollen development ofBrassica napus cv. Topas. Protoplasma 183, 67–76 (1994). https://doi.org/10.1007/BF01276814
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01276814