Summary
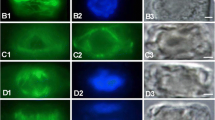
The cytoskeleton in the siphonous, marine green algaAcetabularia is visualized by immunocytochemistry using antibodies against plant alfa tubulin and animal smooth muscle actin. In the vegetative phase of the life cycle, when the cell grows a cylindrical stalk and until the reproductive cap is completed, actin forms continuous, parallel bundles that extend through the entire length of the stalk and cap rays respectively. Microtubules (MTs) cannot be detected until the primary nucleus, located in the rhizoid of the giant cell, divides to form thousands of secondary nuclei. MTs can then be seen radiating from each secondary nucleus that is encountered in the stalk on its migration upwards into the cap rays. They are oriented mostly parallel to the long axis of the cell. At arrival in the cap rays up to the “white spot” stage, when nuclei assume equidistant positions in the cap ray cytoplasm, a radiating system of MTs forms around each nucleus and dramatically increases until impressive radial arrays have developed. This phase coincides with a disappearance of actin bundles in the cap rays, but they are retained in the stalk cytoplasm. Shortly after that additional MTs appear around the disk like partitions of cap ray cytoplasm. Concomitantly, bundles of actin reappear colinearly with the circumferrential MTs eventually forming complete rings around each disk of cap ray cytoplasm. During this process the compartments of the future cysts are gradually bulging outwards and simultaneously the rings of actin sink inwards until domes are formed with the nuclei fixed in the top centers of the domes. At this stage the peripheral areas of the radiating MT systems around the nuclei start to break down, whereas the circumferrential MT systems remain intact. Subsequently, the rings of both actin and MTs decrease in diameter, and finally contract to a spot opposite the nucleus, while the cysts continue to develop their oval shape. After the cysts have become separated, they round up and enter several rounds of nuclear divisions. MTs form short radial arrays around each nucleus with minor changes due to a reduction of MTs during division followed by a reappearance after completion of each division. Actin is rearranged in the cysts to a cortical network of randomly oriented, short bundles, that is maintained until gamete formation sets in.
These findings accentuate the involvement of Cytoskeletal elements in the key steps of morphogenesis inAcetabularia to an extent that is unknown in higher plants.
Similar content being viewed by others
References
Berger S, Schweiger Hg (1980) Techniques for the study of nucleo-cytoplasmic interrelationships. In:Gaunt E (ed) Handbook of phycological methods. University Press, Cambridge, pp 47–57
—,Herth W, Franke WW, Falk H, Spring H, Schweiger HG (1975) Morphology of the nucleocytoplasmic interactions during the development ofAcetabularia cells. Protoplasma 84: 233–256
Bonotto S, Lurquin P, Mazza A (1976) Recent advances in the research on the marine algaAcetabularia. Adv Mar Biol 14: 123–250
Beth K (1943) Ein- und zweikernige Transplantate zwischenAcetabularia mediterranea undAcicularia schenckii. Z Abstammungs- und Vererbungslehre 81: 271–312
Cyr R, Tochi L, Fosket De (1984) Immunological studies on plant tubulin isolated from divers cell lines. J Cell Biol 99: 41a
Dazy A-C, Hoursiangou-Neubrun D, Sauron M-E (1981) Evidence for actin in the marine algaAcetabularia mediterranea. Biol Cell 41: 235–238
Franke WW, Kartenbeck J, Spring H (1976) Involvement of bristle coat structures in surface membrane formation and membrane interactions during coenocytotomic cleavage in caps ofAcetabularia mediterranea. J Cell Biol 71: 196–206
—,Spring H, Kartenbeck J, Falk H (1977) Cyst formation in some dasycladacean green algae. I. Vesicle formations during coenocytotomy inAcetabularia mediterranea. Cytobiology 14: 229–259
—,Berger S, Falk H, Spring H, Scheer U, Herth W, Trendelenburg MF, Schweiger HG (1974) Morphology of the nucleo-cytoplasmic interactions during the development ofAcetabularia cells. I. The vegetative phase. Protoplasma 82: 249–282
Godward MBE, Beth K, Pacey J (1979) Nuclear division in the cyst, and white spot nuclei preceding cyst formation inAcetabularia wettsteinii. Protoplasma 101: 37–46
Gunning BES, Hardham AR (1982) Microtubules. Ann Rev Plant Physiol 33: 651–698
—,Wick SM (1985) Preprophase bands, phragmoplasts, and spatial control of cytokinesis. J Cell Sci [Suppl] 2: 157–179
Hammerling J (1943) Ein- und zweikernige Transplantate zwischenAcetabularia crenulata undAcetabularia mediterranea. Z Abstammungs- und Vererbungslehre 81: 114–180
— (1963) Nucleocytoplasmic interactions inAcetabularia and other cells. Ann Rev Plant Physiol 14: 65–92
Jackson WT (1982) Actomyosin. In:Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London
Kilmartin JV, Adams AEM (1984) Structural rearrangements of tubulin and actin during the cell cycle of the yeastSaccharomyces. J Cell Biol 98: 922–933
Kloppstech K (1982)Acetabularia. In:Smith H, Grierson D (eds) The molecular biology of plant development, Botanical monographs, vol 18. Blackwell Scientific Publications, Oxford London Edinburgh
Koop H-U (1975) Über den Ort der Meiose beiAcetabularia. Protoplasma 85: 109–114
—,Heunert HH, Schmid R (1977) Division of the primary nucleus ofAcetabularia. Protoplasma 93: 131–134
Koop U (1981) Protoplasmic streaming inAcetabularia. Protoplasma 109: 143–157
—,Kiermayer O (1980 a) Protoplasmic streaming in the giant unicellular green algaAcetabularia mediterranea. I. Formation of intracellular transport systems in the course of cell differentiation. Protoplasma 102: 147–166
— — (1980 b) Protoplasmic streaming in the giant unicellular green algaAcetabularia mediterranea. II. Differential sensitivity of movement systems to substances acting on microfilaments and microtubuli. Protoplasma 102: 295–306
Lessard JL, Carlton D, Edelbrock C (1983) Characterization of monoclonal antibodies to muscle actin. Fed Proc 42: 2213
Lloyd CW (1984) Toward a dynamic helical model for the influence of microtubules an wall patterns in plants. Int Rev Cytol 86: 1–51
—,Clayton L, Dawson PJ, Doonan JH, Hulme JS, Roberts IN, Wells B (1985) The cytoskeleton underlying side walls and cross walls in plants: Molecules and macromolecular assemblies. J Cell Sci [Suppl] 2: 143–155
Menzel D,Schliwa M (1986 a) Motility in the siphonous green algaBryopsis. I. Spatial organization of the cytoskeleton and organelle movements. Eur J Cell Biol 40: (in press)
- - (1986 b) Motility in the siphonous green algaBryopsis. II. Chloroplast movement requires organized arrays of both microtubules and actin filaments. Eur J Cell Biol 40: (in press)
Neuhaus-URL G, Schweiger H-G (1984) The lid forming apparatus in cysts of the green algaAcetabularia mediterranea. Protoplasma 122: 120–124
Parthasarathy MV (1985) Actin network as a normal component in many vascular plant cells. Am J Bot 72: 1318–1323
Pickett-Heaps JD, McDonald K, Tippit DH (1975) Cell division in the pennate diatomeDiatoma vulgare. Protoplasma 86: 205–242
Quader H,Deichgraber G,Schnepf E (1986) The cytoskeleton ofColaea seed hairs: Patterning during cell wall differentiation. Planta, (in press)
Sanger JM, Sanger JW (1980) Banding and polarity of actin filaments in interphase and cleaving cells. J Cell Biol 86: 568–575
Saranno T, Pickett-Heaps JD (1982) Directionally controlled spindle disassembly after mitosis in the diatomPinnularia. Eur J Cell Biol 26: 234–243
Schliwa M (1984) Mechanisms of intracellular organelle transport. In:Shay JW (ed) Cell and muscle motility, vol 5. Plenum Publishing Corporation, New York London
Schnepf E, Deichgraber G, Drebes G (1978) Development and ultrastructure of the marine, parasitic oomycete,Lagenisma coscinadisci Drebes (Lagenidiales): Formation of the primary Zoospores and their release. Protoplasma 94: 263–280
Schroeder TE (1981) Interrelations between the cell surface and the cytoskeleton in cleavin sea urchin eggs. In:Poste G, Nicolson GL (eds) Cell surface reviews, vol 7. North-Holland Publishing Company, Amsterdam New York, pp 170–216
Schulze KL (1939) Cytologische Untersuchungen anAcetabularia mediterranea undAcetabularia wettsteinii. Arch Protistenkunde 92: 179–225
Schweiger HG, Berger S (1981) Pattern formation inAcetabularia. In:Kiermayer O (ed) Cytomorphogenesis in plants. Springer, Wien New York
—,Dehm P, Berger S (1977) Culture conditions forAcetabularia. In:Woodcock CLF (ed) Progress inAcetabularia research. Academic Press, New York, pp 319–330
—,Werz G, Reuter W (1969) Tochtergenerationen von heterologen Implantaten beiAcetabularia. Protoplasma 68: 354–356
Trendelenburg MF, Spring H, Scheer U, Franke WW (1974) Morphology of nucleolar cistrons in a plant cell,Acetabularia mediterranea. Proc Natl Acad Sci USA 71: 3626–3630
Tucker JB (1971) Microtubules and a contractile ring of microfilaments associated with a cleavage furrow. J Cell Sci 8: 557–571
Werz G (1955) Kernphysiologische Untersuchungen anAcetabularia. Planta 46: 113–153
Werz G (1968) Plasmatische Formbildung als Voraussetzung für die Zellwandbildung bei der Morphogenese vonAcetabularia. Protoplasma 65: 81–96
— (1969) Wirkung von Colchicine auf die Morphogenese inAcetabularia. Protoplasma 67: 67–78
— (1974) Fine structural aspects of morphogenesis inAcetabularia. Int Rev Cytol 38: 319–167
Williamson RE (1976) Actin and motility in plant cells. In:Perry SV et al (eds) Contractile systems in non-muscle tissues. Elsevier/North-Holland Biomedical Press, Amsterdam
Woodcock CLF (1971) The anchoring of nuclei by microtubules inAcetabularia. J Cell Sci 8: 611–621
-Miller GF (1973) Ultrastructural features of the life cycle ofAcetabularia. II. Events associated with the division of the primary nucleus and the formation of cysts
Zimmer B, Werz G (1981) Cytoskeletal elements and their involvement inPolyphysa (Acetabularia) protoplast differentiation. Cytoskeletal modifiers and concanavalin A-mediated effects. Exp Cell Res 131: 105–113
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Menzel, D. Visualization of cytoskeletal changes through the life cycle inAcetabularia . Protoplasma 134, 30–42 (1986). https://doi.org/10.1007/BF01276373
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01276373