Summary
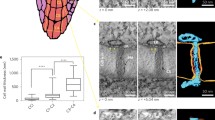
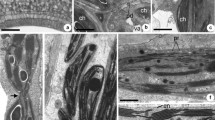
Unripe avocado fruit (Persea americana Mill. cv Hass) were held at 6 °C either in air or in an atmosphere with 100 PPM ethylene and were assessed for chilling injury after one and two weeks. Injury did not occur in any fruit after one week. After two weeks, the fruit in air were still uninjured, but the fruit subjected to ethylene exhibited chilling injury. When the uninjured fruit (both air-treated for one and two weeks and ethylene-treated for one week) were allowed to warm to room temperature before freezing for freeze fracture electron microscopy, replicas revealed membranes with a randomly dispersed pattern of intramembranous particles (IMPs). However, when these uninjured fruit were frozen for freeze fracture without warming, particle-free domains were visible in the plasmalemma. The membranes of the ethylene-treated, chilling-injured (2 weeks) fruit, on the other hand, contained particle-depleted regions in the plasmalemma of fruit frozen not only from 6 °C but also in those allowed to warm to room temperature before freezing for freeze fracture. These particle depleted microdomains were not seen in fruit kept continuously at room temperature (20 °C), even in the presence of high levels of endogenous ethylene which is produced during normal ripening. We suggest these particle-depleted microdomains formed in the fruit frozen for freeze fracture from low temperatures and in the chilling-injured fruit to be due to lateral phase separations of the membrane components, possibly due to an increase in the viscosity of some membrane lipids, leading to the formation of microdomains of gel phase lipid in the plane of the membrane. These phase separations appear to be initially reversible by raising the temperature, however, this reversibility is apparently lost after injury has occurred. With regard to the cause of chilling injury in avocados, we suggest that some secondary effect is involved due to the long term presence of gel phase lipids in the membrane.
Similar content being viewed by others
References
Aloia RC, Rouser G (1975) The effect of temperature changes on lipids and proteins of biological membranes. In:Perkins EG, Witting LA (eds) Modification of lipid metabolism. Academic Press, New York, pp 225–246
Barber RF, Thompson JE (1980) Senescence-dependent increase in the permeability of liposomes prepared from bean cotyledon membranes. J Exp Bot 31: 1305–1313
Biale JB, Young RE (1971) The avocado pear. In:Hulme AC (ed) The biochemistry of fruits and their products, vol 2. Academic Press, London, pp 1–63
Bishop DG (1983) Functional role of plant membrane lipids. In:Thomson WW, Mudd JB, Gibbs M (eds) Biosynthesis and function of plant lipids. Waverly Press, Baltimore, pp 81–103
—,Kendrick JR, Bayston JH, Macpherson AS, Johns SR, Willing RI (1979) The influence of fatty acid unsaturation on fluidity and molecular packing of chloroplast membrane lipids. In:Lyons JM, Graham D, Raison JK (eds) Low temperature stress in crop plants: the role of the membrane. Academic Press, New York, pp 375–389
Branton D, Bullivant S, Gilula NB, Karnovsky MJ, Moore H, Muhlethaler K, Northcote Dh, Packer L, Satir B, Satir P, Speth V, Staehelin LA, Steere RL, Weinstein RS (1975) Freeze-etching nomenclature. Science 190: 54–56
Chaplin GR, Wills RBH, Graham D (1983) Induction of chilling injury in stored avocados with exogenous ethylene. HortSci 18: 952–953
Christiansen MN, St. John JB (1981) The nature of chilling injury and its resistance in plants. In:Olien CR, Smith MN (eds) Analysis and improvement of plant cold hardiness. CRC Press, Boca Raton, pp 1–16
Couey HM (1982) Chilling injury of crops of tropical and subtropical origin. HortSci 17: 162–165
Haest CWM, DeGier J, van Es GA, Verkleij AJ, van Deenen LLM (1972) Fragility of the permeability barrier ofEscherichia coli. Biochim Biophys Acta 288: 43–53
Ilker R, Waring AJ, Lyons JM, Breidenbach RW (1976) The cytological responses of tomato-seedling cotyledons to chilling and the influence of membrane modifications upon these responses. Protoplasma 90: 229–252
James R, Branton D (1973) Lipid- and temperature-dependent structural changes inAcholeplasma laidlawii cell membranes. Biochim Biophys Acta 323: 378–390
Kimball SL, Salisbury FB (1973) Ultrastructural changes of plants exposed to low temperatures. Am J Bot 60: 1028–1033
Kitajima Y, Thompson Jr GA (1977) Differentiation of food vacuolar membrane during endocytosis inTetrahymena. J Cell Biol 75: 436–445
Lees GL, Thompson JE (1980) Lipid composition and molecular organization in plasma membrane-enriched fractions from senescing cotyledons. Physiol Plant 49: 215–221
Lieberman M, Craft CC, Audia WV, Wilcox MS (1958) Biochemical studies of chilling injury in sweet potatoes. Plant Physiol 33: 307–311
Lynch DV, Thompson Jr, GA (1984 a) Microsomal phospholipid molecular species alterations during low temperature acclimation inDunaliella. Plant Physiol 74: 193–197
— — (1984 b) Chloroplast phospholipid molecular species alterations during low temperature acclimation inDunaliella. Plant Physiol 74: 198–203
Lyons JM (1973) Chilling injury in plants. Ann Rev Plant Physiol 24: 445–466
—,Graham D, Raison JK (1979) Low temperature stress in crop plants: the role of the membrane. Academic Press, New York
—,Raison JK, Steponkus PL (1979) The plant membrane in response to low temperature: an overview. In:Lyons JM, Graham D, Raison JK (eds) Low temperature stress in crop plants: the role of the membrane. Academic Press, New York, pp 1–24
—,Wheaton TA, Pratt HK (1964) Relationship between the physical nature of mitochondrial membranes and chilling sensitivity in plants. Plant Physiol 39: 262–268
Martin B (1986) Arrhenius plots and the involvement of thermotropic phase transitions of the thylakoid membrane in chilling impairment of photosynthesis in thermophilic higher plants. Plant Cell Environ 9: 323–331
McKersie BD, Thompson JE (1977) Lipid crystallization in senescent membranes from cotyledons. Plant Physiol 59: 803–807
Moeller CH, Mudd JB, Thomson WW (1981) Lipid phase separations and intramembranous particle movements in the yeast tonoplast. Biochim Biophys Acta 643: 376–386
Niki T, Yoshida S, Sakai A (1978) Studies on chilling injury in plant cells. I. Ultrastructural changes associated with chilling injury in callus tissues ofCornus stonoifera. Plant Cell Physiol 19: 139–148
O'Neill SD, Leopold AC (1982) An assessment of phase transitions in soybean membranes. Plant Physiol 70: 1405–1409
Papahadjopoulos DM, Jacobson K, Nir S, Isac T (1973) Phase transition and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta 311: 330–348
Platt-Aloia KA, Thomson WW (1976) An ultrastructural study of two forms of chilling-induced injury to the rind of grapefruit (Citrus paradisi Macfed.). Cryobiol 13: 95–106
— — (1981) Ultrastructure of the mesocarp of mature avocado fruit and changes associated with ripening. Ann Bot 48: 451–465
— — (1982) Freeze-fracture of intact plant tissues. Stain Technol 57: 327–334
Quinn PJ, Williams WP (1978) Plant lipids and their role in membrane function. Prog Biophys Mol Biol 34: 104–173
Raison JK (1974) A biochemical explanation of low temperature stress in tropical and subtropical plants. In:Bieleski RL, Ferguson AR (eds) Mechanisms of regulation of plant growth, Bulletin 12. Roy Sol NZ, Wellington, pp 487–497
—,Chapman EA (1976) Membrane phase changes in chilling-sensitiveVigna radiata and their significance to growth. Aust J Plant Physiol 3: 291–299
—,Lyons JM, Thomson WW (1971) The influence of membranes on the temperature-induced changes in the kinetics of some respiratory enzymes of mitochondria. Arch Biochem Biophys 142: 83–90
—,Orr GR (1986 a) Phase transitions in thylakoid polar lipids of chilling-sensitive plants. Plant Physiol 80: 638–645
— — (1986 b) Phase transitions in liposomes formed from the polar lipids of mitochondria from chilling-sensitive plants. Plant Physiol 81: 807–811
—,Wright LC (1983) Thermal phase transitions in the polar lipids of plant membranes. Their induction by disaturated phospholipids and their possible relation to chilling injury. Biochim Biophys Acta 731: 69–78
Robards AW, Clarkson DT (1984) Effects of chilling temperatures on root cell membranes as viewed by freeze-fracture electron microscopy. Protoplasma 122: 75–85
Shechter E, Letellier L, Gulik-Kuzywiki T (1974) Relations between structure and function in cytoplasmic membrane vesicles isolated from anE. coli fatty acid auxotroph. Eur J Biochem 49: 61–76
Singer MA (1983) What have permeability experiments revealed about the structure of phospholipid bilayer membranes? In:Bangham AD (ed) Liposome letters. Academic Press, London, pp 105–112
Thompson GA, Jr (1980) Regulation of membrane fluidity during temperature acclimation byTetrahymena pyriformis. In:Kates M, Kuksis A (eds) Membrane fluidity: biophysical techniques and cellular regulation. Humana Press, Clifton, New Jersey, pp 381–397
Thompson JE, Mayfield CI, Inniss WE, Butler DE, Kruuv J (1978) Senescence-related changes in the lipid transition temperature of microsomal membranes from algae. Physiol Plant 43: 114–120
Toivio-Kinnukan MA, Chen HH, Li PH, Stuchnoff C (1981) Plasma membrane alterations in callus tissues of tuber-bearingSolanum species during cold acclimation. Plant Physiol 67: 478–483
Wunderlich F, Ronai A, Speth V, Seelig J, Blume A (1975) Thermotropic lipid clustering inTetrahymena membranes. Biochem 14: 3730–3735
Yoshida S, Niki T, Sakai A (1979) Possible involvement of the tonoplast lesion in chilling injury of cultured plant cells. In:Lyons JM, Graham D, Raison JK (eds) Low temperature stress in crop plants: the role of the membrane. Academic Press, New York, pp 275–290
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Platt-Aloia, K.A., Thomson, W.W. Freeze fracture evidence for lateral phase separations in the plasmalemma of chilling-injured avocado fruit. Protoplasma 136, 71–80 (1987). https://doi.org/10.1007/BF01276356
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01276356