Abstract
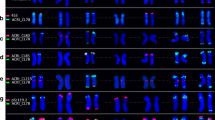
A digoxigenin-labelled 5S rDNA probe (pTa-794) and a rhodamine-labelled 18S-5.8S-25S rDNA probe (pTa71) were used for double-target in-situ hybridization to root-tip metaphase, prophase and interphase chromosomes of cultivated beet,Beta vulgaris L. After in-situ hybridization with the 18S-5.8S-25S rDNA probe, one major pair of sites was detected which corresponded to the secondary constriction at the end of the short arm of chromosome 1. The two rDNA chromosomes were often associated and the loci only contracted in late metaphase. In the majority of the metaphase plates analyzed, we found a single additional minor hybridization site with pTa71. One pair of 5S rRNA gene clusters was localized near the centromere on the short arm of one of the three largest chromosomes which does not carry the 18S-5.8S-25S genes. Because of the difficulties in distinguishing the very similarly-sizedB. vulgaris chromosomes in metaphase preparations, the 5S and the 18S-5.8S-25S rRNA genes can be used as markers for chromosome identification. TwoXbaI fragments (pXV1 and pXV2), comprising the 5S ribosomal RNA gene and the adjacent intergenic spacer, were isolated. The two 5S rDNA repeats were 349 bp and 351 bp long, showing considerable sequence variation in the intergenic spacer. The use of fluorescent in-situ hybridization, complemented by molecular data, for gene mapping and for integrating genetic and physical maps of beet species is discussed.
Similar content being viewed by others
References
Barciszewska, MZ, Mashkova, TD, Kisselev, LL, Barciszewski, J (1986) The nucleotide sequence of 5S ribosomal genes from two plants: rape and white beet. Nucleic Acids Res 15:363
Barzen E, Mechelke W, Ritter E, Seitzer JF, Salamini F (1992) RFLP markers for sugar beet breeding: chromosomal linkage maps and location of major genes for rhizomania resistance, monogermyand hypocotyl colour. Plant J 2:601–611
Bennett MD, Smith JB (1976) Nuclear DNA amounts in angiosperms. Phil Trans R Soc Lond B 274: 227–274
Bosemark NO, Bormotov VE (1971) Chromosome morphology in a homozygous line of sugar beet. Hereditas 69:205–212
Butterfass, T (1963) Die Chloroplastenzahlen in verschiedenartigen Zellen trisomer Zuckerrüben (Beta vulgaris L.). Z Bot 52:46–77
de Jong H (1981) Investigation into chromosome morphology of sugar beet and related wild species. PhD thesis, University of Amsterdam
de Jong H, de Bock TSM (1978) Use of haploids ofBeta vulgaris L. for the study of orcein- and Giemsa-stained chromosomes. Euphytica 27:41–47
Ellis THN, Lee D, Thomas CM, Simpson PR, Cleary WG, Newman MA, Burcham KWG (1988) 5S rRNA genes inPisum: sequence, long range and chromosomal organization. Mol Gen Genet 214:333–342
Flavell RB (1986) The structure and control of expression of ribosomal RNA genes. Oxford Surveys Plant Mol Cell Biol 3:251–274
Gerlach WL, Bedbrook JR (1979) Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res 7:1869–1885
Gerlach WL, Dyer TA (1980) Sequence organization of the repeating units in the nucleus of wheat which contain 5S rRNA genes. Nucleic Acids Res 8:4851–4855
Hemleben V, Werts D (1988) Sequence organization and putative regulatory elements in the 5S rRNA genes of two higher plants (Vigna radiata andMatthiola incana). Gene 62: 165–169
Heslop-Harrison JS, Schwarzacher T, Anamthawat-Jónsson K, Leitch AR, Shi M, Leitch IJ (1991)In-situ hybridization with automated chromosome denaturation. Technique 3:109–116
Ingle J, Timmis JN, Sinclair J (1975) The relationship between satellite desoxyribonucleic acid, ribosomal ribonucleic acid redundancy, and genome size in plants. Plant Physiol 55:496–501
Lapitan NLV, Ganal MW, Tanksley SD (1991) Organization of the 5S ribosomal RNA genes in the genome of tomato. Genome 34:509–514
Larsen K (1977) Self-incompatibility inBeta vulgaris L. 1. Four gametophytic complementary S-loci in sugar beet. Hereditas 85:227–248
Lehfer H, Busch W, Martin R, Herrmann RG (1993) Localization of the B-hordein locus on barley chromosomes using fluorescence in-situ hybridization. Chromosoma 102:428–432
Leitch IJ, Heslop-Harrison JS (1992) Physical mapping of the 18S-5.8S-26S rRNA genes in barley bysitu hybridization. Genome 35:1013–1018
Leitch IJ, Heslop-Harrison JS (1993) Physical mapping of four sites of 5S ribosomal DNA sequences and one site of the α-amylase 2 gene in barley (Hordeum vulgare). Genome 36:517–523
Leitch AR, Mosgöller W, Shi M, Heslop-Harrison JS (1992) Different pattern of rDNA organization at interphase in nuclei of wheat and rye. J Cell Sci 101:751–757
Leitch AR, Schwarzacher T, Wang ML, Leitch IJ, Surlan-Momirovich G, Moore G, Heslop-Harrison JS (1993) Molecular cytogenetic analysis of repeated sequences in a long term wheat suspension culture. Plant Cell Tissue Org Cult 33:287–296
Maluszynska J, Heslop-Harrison JS (1991) Localization of tandemly-repeated DNA sequences inArabidopsis thaliana. Plant J 1:159–166
Maluszynska J, Heslop-Harrison JS (1993) Physical mapping of rDNA loci inBrassica species. Genome 36:774–781
Mukai Y, Endo TR, Gill BS (1990) Physical mapping of the 5S rRNA multigene family in common wheat. J Heredity 81:290–295
Nakamura C, Tsuchiya T (1982) Pachytene chromosome morphology in diploid sugar beet,Beta vulgaris. Z Pflanzenzuchtg 89:229–244
Nakamura C, Skaracis GN, Romagosa I (1991) Cytogenetics and breeding in sugar beet. In: Tsuchiya T, Gupta PK (eds) Chromosome engineering in plants: genetics, breeding, evolution. Elsevier, Amsterdam Oxford New York Tokyo, pp 295–314
Pillen K, Steinrücken G, Wricke G, Herrmann RG, Jung C (1992) A linkage map of sugar beet (Beta vulgaris L.). Theor Appl Genet 84:129–135
Röder MS, Sorrells ME, Tanksley SD (1992) 5S ribosomal gene clusters in wheat: pulsed-field gel electrophoresis reveals a high degree of polymorphism. Mol Gen Genet 232:215–220
Rogers SO, Bendich AJ (1987) Ribosomal RNA genes in plants: variability in copy number and in the intergenic spacer. Plant Mol Biol 9:509–520
Schmidt T, Heslop-Harrison JS (1993) Variability and evolution of highly-repeated DNA sequences in the genusBeta. Genome 36:1074–1079
Schmidt T, Jung C, Metzlaff M (1991) Distribution and evolution of two satellite DNAs in the genusBeta. Theor Appl Genet 82:793–799
Schmidt T, Boblenz K, Metzlaff M, Kaemmer D, Weising, K, Kahl G (1993) DNA fingerprinting in sugar beet (Beta vulgaris) — identification of double-haploid breeding lines. Theor Appl Genet 85:653–657
Schwarzacher T, Leitch AR, Bennett MD, Heslop-Harrison JS (1989)In-situ localization of parental genomes in a wide hybrid. Ann Bot 64: 315–324
Schwarzacher T, Anamthawat-Jónsson K, Harrison GE, Islam AKMR, Jia JZ, King IP, Leitch AR, Miller TE, Reader SM, Rogers WJ, Shi M, Heslop-Harrison JS (1992) Genomic in-situ hybridization to identify alien chromosomes and chromosome segments in wheat. Theor Appl Genet 84: 778–786
Selker, EU, Morzycha-Wroblewska, E, Steven, JN, Metzenberg, RL (1986) An upstream signal is required for in-vitro transcription ofNeurospora 5S RNA genes. Mol Gen Genet 205:189–192
Specht T, Wolters J, Erdmann VA (1990) Compilation of 5S rRNA and 5S rRNA gene sequences. Nucleic Acids Res (suppl.) 18:2215–2230
Venkateswarlu, K, Lee, SW, Nazar, RN (1991) Conserved upstream sequence elements in plant 5S ribosomal RNA-encoding genes. Gene 105:249–253
Author information
Authors and Affiliations
Additional information
Communicated by F. Mechelke
Rights and permissions
About this article
Cite this article
Schmidt, T., Schwarzacher, T. & Heslop-Harrison, J.S. Physical mapping of rRNA genes by fluorescent in-situ hybridization and structural analysis of 5S rRNA genes and intergenic spacer sequences in sugar beet (Beta vulgaris). Theoret. Appl. Genetics 88, 629–636 (1994). https://doi.org/10.1007/BF01253964
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01253964