Summary
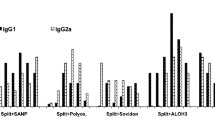
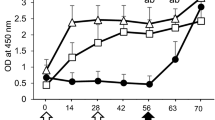
Mice and ferrets were immunized by oral, intranasal or intraperitoneal route with attenuated or inactivated influenza A2/Aichi/68. Production of antibodies in groups immunized orally was lower than in groups immunized intranasally or intraperitoneally when attenuated virus was administered. When inactivated virus was inoculated the serum antibody response was high following intraperitoneal injection, low following intranasal injection and nil following oral administration.
Deaths occurred between the 3rd and the 11th day after intranasal immunization with partially attenuated virus, showing that immunization by the intranasal route necessitates more attenuated strains than by oral or by intraperitoneal routes. Higher doses of vaccine were required by oral than intranasal or intraperitoneal route to confer an equivalent degree of protection.
Similar content being viewed by others
References
Alexsandrova, G. I.,et al.: Testing the safety and effectiveness of oral administration of a live influenza vaccine. Bull. Wld Hlth Org.42, 429–436 (1970).
Boudreault, A., G. Lussier etV. Pavilanis: Caractères biologiques de souches du virus de l'influenza adaptées à 29°C et à 41°C Canad. J. Microbiol.14, 867–874 (1968).
Boudreault, A., G. Boulay, andV. Pavilanis: Immunization against influenza by oral route. Canad. J. publ. Hlth62, 70 (1970).
Expert Committee on Respiratory Virus Diseases, First Report WHO Tech. Report Series No. 170, pp. 1–59 (1959).
Ikic, D.: Experience in Yugoslavia with live influenza vaccine prepared from an attenuated A2/Hong Kong/68 Strain. Bull. Wld Hlth Org.41, 608–609 (1969).
Kilbourne, E. D.: Future influenza vaccines and the use of genetic recombinants. Bull. Wld Hlth Org.41, 643–645 (1969).
Maassab, H. F., T. Francis, Jr., F. M. Davenport, A. V. Hennessy, E. Minuse, andG. Anderson: Laboratory and clinical characteristics of attenuated strains of influenza virus. Bull. Wld Hlth Org.41, 589–594 (1969).
Mills, J. V., andR. M. Chanock: Temperature-sensitive mutants of influenza virus. I. Behavior in tissue culture and in experimental animals. J. infect. Dis.123, 145–157 (1971).
Smorodincev, A. A.: The effivacy of live influenza vaccines. Bull. Wld Hlth Org.41, 585–588 (1969).
Solov'ev, V. D.: The results of controlled observations on the prophylaxis of influenza with interferon. Bull. Wld Hlth Org.41, 683–688 (1969).
Tyrrell, D. A. J., andA. S. Beare: Some studies on the selection and efficiency of live influenza vaccine viruses. Bull. Wld Hlth Org.41, 581–584 (1969).
Webster, R. G.: Antigenic hybrids of influenza A viruses with surface antigens to order. Virology42, 633–642 (1970).
Author information
Authors and Affiliations
Additional information
This work was partially supported by a grant from National Research Council (915).
Rights and permissions
About this article
Cite this article
Boudreault, A., Pavilanis, V. Oral immunization against influenza virus. Archiv f Virusforschung 38, 177–182 (1972). https://doi.org/10.1007/BF01249668
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01249668