Abstract
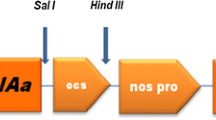
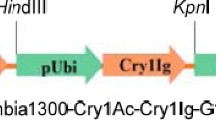
We usedAgrobacterium tumefaciens to transform flowering stalk explants of five genotypes of broccoli with a construct containing the neomycin phosphotransferase gene and aBacillus thuringiensis (Bt) gene [CryIA(c) type] optimized for plant expression. Overall transformation efficiency was 6.4%; 181 kanamycin-resistant plants were recovered. Of the 162 kanamycin-resistant plants tested, 112 (69%) caused 100% morality of 1st-instar larvae of aBt-susceptible diamondback moth strain. Southern blots of some resistant transformants confirmed presence of theBt gene. Selected plants that gave 100% mortality of susceptible larvae allowed survival of a strain of diamondback moth that had evolved resistance toBt in the field. F1 hybrids between resistant and susceptible insects did not survive. Analysis of progeny from 26 resistant transgenic lines showed 16 that gave segregation ratios consistent with a single T-DNA integration. Southern analysis was used to verify those plants possessing a single T-DNA integration. Because these transgenic plants kill susceptible larvae and F1 larvae, but serve as a suitable host for resistant ones, they provide an excellent model for tests ofBt resistance management strategies.
Similar content being viewed by others
References
Arumuganathan K, Earle ED: Estimation of nuclear DNA content of plants by flow cytometry. Plant Mol Biol Rep 9: 229–233 (1991).
Arumuganathan K, Earle ED: Nuclear DNA content of some important plant species. Plant Mol Biol Rep 9: 208–218 (1991).
Bai YY, Mao HZ, Cao XL, Tang T, Wu D, Chen DD, Li WG, Fu WJ: Transgenic cabbage plants with insect tolerance. In: You CBet al. (eds) Biotechnology in Agriculture, pp. 156–159. Kluwer Academic Publishers, Netherlands (1993).
Bottrell DG, Aguda RM, Gould FL, Theunis W, Demayo CG, Magalit VF: Potential strategies for prolonging the usefulness ofBacillus thuringiensis in engineered rice. Korean J Appl Entomol 31: 247–255 (1992).
Cheng J, Bolyard MG, Saxena RC, Sticklen M: Production of insect resistant potato by genetic transformation with a delta-endotoxin fromBacillus thuringiensis var.kurstaki. Plant Sci 81: 83–91 (1992).
Church GM, Gilbert W: Genomic sequencing. Proc Natl Acad Sci USA 81: 1991–1995 (1984).
Dean C, Jones J, Favreau M, Dunsmuir P, Bedbrook J: Influence of flanking sequences on variability in expression levels of an introduced gene in transgenic tobacco plants. Nucl Acids Res 16: 9267–9283 (1988).
Ferro DN: Potential for resistance toBacillus thuringiensis: Colorado potato beetle (Coleoptera: Chysomelidae): a model system. Am Entomol 39: 38–44 (1993).
Fromm M, Armstrong C, Blasingame A, Brown S, Duncan D, Deboer D, Hairston B, Howe A, McCaul S, Neher M, Pajeau M, Parker G, Pershing J, Petersen B, Santino C, Sanders P, Sato S, Sims S, Thorton T: Production of insect resistant corn. J Biol Chem (Suppl. 18A): 77 (1994).
Fry J, Barnason A, Horsch RB: Transformation ofBrassica napus withAgrobacterium tumefaciens based vectors. Plant Cell Rep 6: 321–325 (1987).
Fuchs RL, MacIntosh SC, Dean DA, Greenplate JT, Perlak FJ, Pershing JC, Marrone PG, Fischhoff DA: Quantification ofBacillus thuringiensis insect control protein as expressed in transgenic plants. In: Hickle LA, Fitch WL (eds) Analytical Chemistry ofBacillus thuringiensis, pp. 105–113. American Chemical Society, Washington, DC (1990).
Fujimoto H, Itoh K, Yamamoto M, Kyozuka J, Shimamoto K: Insect resistant rice generated by introduction of a modified∂-endotoxin gene ofBacillus thuringiensis. Bio/technology 11: 1151–1155 (1993).
Gallun RL, Khush GS: Genetic factors affecting the expression and stability of resistance. In: Maxwell FG, Jennings PR (eds) Breeding Plants Resistant to Insects, pp. 64–85. Wiley, New York (1980).
Gamborg OL, Miller RA, Ojima K: Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158 (1968).
Gibbons A: Moths take the field against biopesticides. Science 254: 646 (1991).
Gould F: Simulation models for predicting durability of insect-resistant germplasm: a deterministic diploid, two-locus model. Envir Entomol 15: 1–10 (1986).
Gould F: Genetic engineering, integrated pest management and the evolution of pests. Trends Biotechnol 6: 15–18 (1990).
Hama H, Suzuki K, Tanaka H: Inheritance and stability of resistance toBacillus thuringiensis formulations of the diamondback moth,Plutella xylostella (Linnaeus) (Lepidoptera: Yponomeutidae). Appl Entomol Zool 27: 355–362 (1992).
Hansen WD: Minimum family sizes for genetic experiments. Agron J 51: 711–715 (1959).
Höfte H, Whiteley HR: Insecticidal crystal proteins ofBacillus thuringiensis. Microbiol Rev 53: 242–255 (1989).
Kirsch K, Schmutterer H: Low efficacy of aBacillus thuringiensis (Berl.) formulation in controlling the diamondback moth,Plutella xylostella (L.), in the Philippines. J Appl Entomol 105: 249–255 (1988).
Koziel MG, Beland GL, Bowman C, Carozzi NB, Crenshaw R, Crossland L, Dawson J, Desai N, Hill M, Kadwell S, Launis K, Lewis K, Maddox D, McPherson K, Meghji MR, Merlin E, Rhodes R, Warren GW, Wright M, Evola SV: Field performance of elite transgenic maize plants expressing an insecticidal protein derived fromBacillus thuringiensis. Bio/technology 11: 194–200 (1993).
Mallet J, Porter P: Preventing insect adaptation to insect-resistant crops: are seed mixtures or refugia the best strategy? Proc R Soc Lond B 250: 165–169 (1992).
Matzke M, Matzke AJM: Genomic imprinting in plants: parental effects andtrans-inactivation phenomena. In: Briggs WR, Jones RL, Walbot V (eds) Annu Rev Plant Physiol Plant Mol Biol 44: 53–76 (1993).
McGaughey WH, Beeman RW: Resistance toBacillus thuringiensis in colonies of Indian meal moth and almond moth (Lepidoptera: Pyralidae). J Econ Entomol 81: 28–33 (1990).
McGaughey WH, Whalon ME: Managing insect resistance toBacillus thuringiensis toxins. Science 258: 1451–1455 (1992).
Murashige T, Skoog F: A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15: 473–497 (1962).
Perlak FJ, Deaton RW, Armstrong TA, Fuchs RL, Sims SR, Greenplate JT, Fischhoff DA: Insect resistant cotton plants. Bio/technology 8: 939–943 (1990).
Perlak FJ, Fuchs RL, Dean DA, McPherson SL, Fischhoff DA: Modification of the coding sequence enhances plant expression of insect control protein genes. Proc Natl Acad Sci USA 88: 3324–3328 (1991).
Roush RT: Managing pests and their resistance toBacillus thuringiensis: can transgenic crops be better than sprays? Biocontrol Sci Technol 4: 501–516 (1994).
Rowe GE, Margaritis A: Bioprocess developments in the production of bioinsecticides byBacillus thuringiensis. CRC Crit Rev Biotechnol 6: 87–127 (1987).
Shelton AM, Cooley RJ, Kroening MK, Wilsey WT, Eigenbrode SD: Comparative analysis of two rearing procedures for diamondback moth,Plutella xylostella (Lepidoptera: Plutellidae). J Entomol Sci 26: 17–26 (1991).
Shelton AM, Robertson JL, Tang JD, Perez C, Eigenbrode SD, Preisler HK, Wilsey WK, Cooley RJ: Resistance of diamondback moth toBacillus thuringiensis subspecies in the field. J Econ Entomol 86: 697–705 (1993).
Shure W, Wessler S, Federoff N: Molecular identification and isolation of theWaxy locus in maize. Cell 35: 235–242 (1983).
Southern EM: Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98: 503 (1975).
Stone TB, Sims SR, Marrone PG: Selection of tobacco budworm for resistance to a genetically engineeredPseudomonas fluorescens containing the delta-endotoxin ofBacillus thuringiensis subsp.kurstaki. J Invert Path 53: 228–234 (1989).
Sullivan J, Lagrimini LM: Transformation ofLiquidambar stryraciflua usingAgrobacterium tumefaciens. Plant Cell Rep 12: 303–306 (1993).
Tabashnik BE: Evolution of resistance toBacillus thuringiensis. Ann Rev Entomol 39: 47–79 (1994).
Tabashnik BE: Delaying insect adaptation to transgenic crops: seed mixtures and refugia reconsidered. Proc R Soc Lond Ser. B 255: 7–12 (1994).
Tabashnik BE, Cushing N, Finson N, Johnson MW: Field development of resistance toBacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J Econ Entomol 83: 1671–1676 (1990).
Tabashnik BE, Finson N, Johnson MW: Managing resistance toBacillus thuringiensis: lessons from the diamondback moth (Lepidoptera: Plutellidae). J Econ Entomol 84: 49–55 (1991).
Tabashnik BE, Schwartz JM, Finson N, Johnson MW: Inheritance of resistance toBacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J Econ Entomol 85: 1046–1055 (1992).
Talekar NS, Shelton AM: Biology, ecology and management of the diamondback moth. Annu Rev Entomol 38: 275–301 (1993).
Toriyama K: Transformation ofBrassica species with self-incompatibility gene. In: Oono K, Hirabayashi T, Kikuchi S, Handa H, Kajiwara K (eds) Plant Tissue Culture and Gene Manipulation for Breeding and the Formation of Phytochemicals, pp. 165–171. NIAR, Japan (1992).
Vaeck M, Reynaerts A, Höfte H, Jansens S, De Beuckeleen M, Dean C, Zabeau M, Van Montagu M, Leemans J: Transgenic plants protected from insect attack. Nature 328: 33–37 (1987).
Weide R, Koornneef M, Zabel P: A simple, nondestructive spraying assay for the detection of an active kanamycin resistance gene in transgenic tomato plants. Theor Appl Genet 78: 169–172 (1989).
Whalon ME, Miller DL, Hollingworth RM, Grafius EJ, Miller JR: Selection of a Colorado potato beetle (Coleoptera: Chrysomelidae) strain resistant toBacillus thuringiensis. J Econ Entomol 86: 226–233 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Metz, T.D., Roush, R.T., Tang, J.D. et al. Transgenic broccoli expressing aBacillus thuringiensis insecticidal crystal protein: Implications for pest resistance management strategies. Mol Breeding 1, 309–317 (1995). https://doi.org/10.1007/BF01248408
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01248408