Summary
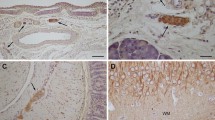
Growth-associated protein, GAP-43 was initially described as a neuron-specific molecule thought to play a critical role in axonal growth and regeneration. However, it is also expressedin vitro in certain CNS glia, Schwann cell precursors and non-myelinating Schwann cells. In this paper, we report the subcellular localization of GAP-43in vivo in chronically-denervated Schwann cells in the distal stumps of previously transected rat sciatic nerve. We have used a progressive lowering of temperature method combined with the non-polar acrylic resin Lowicryl HM20 and a post-embedding labelling regime to visualize the distribution of GAP-43, S-100 (marker for Schwann cells), RT97 and NF68 (markers for different subunits of the neurofilament molecule). We report that (1) the smallest calibre regrowing axons were GAP-43-positive, sometimes NF68-positive but always RT97-negative; (2) regenerating myelinated axons and larger unmyelinated axons (> 0.7 μm diameter) were NF68-positive, RT97-positive but GAP-43-negative; (3) cytoplasmic processes within Schwann cell basal lamina tubes in the distal stumps were S-100-positive, GAP-43-positive but RT97- and NF68-negative. The similar localization of GAP-43 within regrowing axons and denervated Schwann cells suggests that GAP-43 may function similarly in both situations, and may thus be involved in motility and/or elongation of axons and Schwann cells during regeneration.
Similar content being viewed by others
References
Abernethy, D. A., Rud, A. &Thomas, P. K. (1992) Neurotropic influence of the distal stump of transected peripheral nerve on axonal regeneration: absence of topographic specificity in adult nerve.Journal of Anatomy 180, 395–100.
Basi, G. S., Jacobson, R. D., Virag, I., Schilling, J. &Skene, J. H. P. (1987) Primary structure and transcriptional regulation of GAP-43, a protein associated with nerve growth.Cell 49, 785–91.
Bendayan, M. (1982) Double immunocytochemical labelling applying the protein A-gold technique.Journal of Histochemistry and Cytochemistry 30, 81–5.
Benowitz, L. I. &Routtenberg, A. (1987) A membrane phosphoprotein associated with neural development, axonal regeneration, phospholipid metabolism, and synaptic plasticity.Trends in Neuroscience 10, 527–32.
Biffo, S., Verhaagen, J., Schrama, L. H., Schotman, P., Danho, W. &Margolis, F. L. (1990) B-50/GAP-43 expression correlated with process outgrowth in the embryonic mouse nervous system.European Journal of Neuroscience 2, 487–99.
Brockes, J. P., Fields, K. P. &Raff, M. C. (1979) Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve.Brain Research 165, 105–18.
Carlemalm, E., Garavito, R. M. &Villiger, W. (1982) Resin development for electron microscopy and an analysis of embedding at low temperature.Journal of Microscopy 126, 123–43.
Cohen, J. &Johnson, A. R. (1991) Differential effects of laminin and merosin on neurite outgrowth by developing retinal ganglion cells.Journal of Cell Science Suppl15, 1–7.
Curtis, R., Hardy, R., Reynolds, R., Spruce, B. A., &Wilkin, G. P. (1991) Down-regulation of GAP-43 during oligodendrocyte development and lack of expression by astrocytesin vivo: implications for macroglial differentiation.European Journal of Neuroscience 3, 876–86.
Curtis, R., Stewart, H. J. S., Hall, S. M., Wilkin, G. P., Mirsky, R. &Jessen, K. R. (1992) GAP-43 is expressed by non-myelin-forming Schwann cells of the peripheral nervous system.Journal of Cell Biology 116, 1455–64.
Da Cunha, A. &Vitkvić, L. (1990) Regulation of immunoreactive GAP-43 expression in rat cortical macroglia is cell type specific.Journal of Cell Biology 111, 209–15.
Degraan, P. N. E., Van Hooff, C. O. M., Tilly, B. C., Oestreicher, A. B., Schotman, P. &Gispen, W. H. (1985) Phosphoprotein B-50 in nerve growth cones from fetal rat brain.Neuroscience Letters 61, 235–41.
Deloulme, J.-C., Janet, T., Au, D., Storm, D. R., Sensenbrenner, M. &Baudier, J. (1990) Neuromodulin (GAP43): a neuronal protein kinase C substrate is also present in 0-2A glial cell lineage. Characterisation of neuromodulin in secondary cultures of oligodendrocytes and comparison with the neuronal antigenJournal of Cell Biology 111, 1559–69.
Difiglia, M., Roberts, R. C. &Benowitz, L. I. (1990) Immunoreactive GAP-43 in the neuropil of adult rat neostriatum: localization in unmyelinated fibres, axon terminals and dendritic spines.Journal of Comparative Neurology 302, 992–1001.
Doster, S. K., Lozano, A. M., Aguayo, A. J. &Willard, M. B. (1991) Expression of the growth-associated protein GAP-43 in adult rat retinal ganglion cells following axon injury.Neuron 6, 635–47.
Gorgels, T. G. M. F., Campagne, M. Van L., Oestreicher, A. B., Gribnau, A. A. M. &Gispen, W. H. (1989) B-50/GAP43 is localized at the cytoplasmic side of the plasma membrane in developing and adult rat pyramidal tract.Journal of Neuroscience 9, 3861–9.
Goslin, K., Schreyer, D. J., Skene, J. P. H. &Banker, G. (1988) Development of neuronal polarity: GAP-43 distinguishes axonal from dendritic growth cones.Nature 336, 672–4.
Holton, B. &Weston, J. A. (1982a) Analysis of glial cell differentiation in peripheral nervous tissue. I. S100 accumulation in quail embryo spinal ganglion cultures.Developmental Biology 89, 64–71.
Holton, B. &Weston, J. A. (1982b) Analysis of glial cell differentiation in peripheral nervous tissue. II. Neurons promote S100 synthesis by purified glial precursor cell populations.Developmental Biology 89, 72–81.
Isobe, T., Ishioka, T. &Okuyama, T. (1981) Structural relation of two S-100 proteins in bovine brain, subunit composition of S-100a protein.European Journal of Biochemistry 115, 469–74.
Isobe, T., Takahashi, K. &Okuyama, T. (1984a) S-100 protein is present in neurons of the central and peripheral nervous system.Journal of Neurochemistry 43, 1494–6.
Isobe, T., Ichimori, K., Nakajima, T. &Okuyama, T. (1984b) The α subunit of S-100 protein is present in tumor cells of brain malignant melanoma, but not in schwannoma.Brain Research 294, 381–4.
Jacobson, R. D., Virag, I. &Skene, J. H. P. (1986) A protein associated with axon growth, GAP-43 is widely distributed and developmentally regulated in rat CNS.Journal of Neuroscience 6, 1843–55.
Kalil, K. &Skene, J. H. P. (1986) Elevated synthesis of an axonally transported protein correlates with axon out-growth in normal and injured pyramidal tracts.Journal of Neuroscience 6, 2563–70.
Kato, K. &Satoh, T. (1983) Changes in the concentration of enolase isozymes and S-100 protein in degenerating and regenerating rat sciatic nerve.Journal of Neurochemistry 40, 1076–81.
Lankford, K., Cypher, C. &Letourneau, P. (1990) Nerve growth cone motility.Current Opinion in Cell Biology 2, 80–5.
Mata, M., Alessi, D. &Fink, D. J. (1990) S100 is preferentially distributed in myelin-forming Schwann cells.Journal of Neurocytology 19, 432–42.
Meiri, K. F., Pfenninger, K. H. &Willard, M. B. (1986) Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones.Proceedings of the National Academy of Sciences (USA) 83, 3537–41.
Meiri, K. F., Willard, M. &Johnson, M. I. (1988) Distribution and phosphorylation of the growth-associated protein GAP-43 in regenerating sympathetic neurons in culture.Journal of Neuroscience 8, 2571–81.
Meiri, K. F. &Gordon-Weeks, P. R. (1990) GAP-43 in growth cones is associated with areas of membrane that are tightly bound to substrate and is a component of a membrane skeleton subcellular fraction.Journal of Neuroscience 10, 256–66.
Morris, J. H., Hudson, A. R. &Weddell, G. (1972a) A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. I. The traumatic degeneration of myelin in the proximal stump of the divided nerve.Zeitschrift für Zellforschung und mikroskopische Anatomie 124, 76–102.
Morris, J. H., Hudson, A. R. &Weddell, G. (1972b) A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. II. The development of the ‘regenerating unit’.Zeitschrift für Zellforschung und mikroskopische Anatomie 124, 103–30.
Morris, J. H., Hudson, A. R. &Weddell, G. (1972c) A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. III. Changes in the axons of the proximal stump.Zeitschrift für Zellforschung und mikroskopische Anatomie 124, 131–64.
Morris, J. H., Hudson, A. R. &Weddell, G. (1972d) A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. IV. Changes in fascicular microtopography, perineurium and endoneurial fibroblasts.Zeitschrift für Zellforschung und mikroskopishe Anatomie 124, 165–203.
Neuberger, T. J. &Cornbrooks, C. J. (1989) Transient modulation of Schwann cell antigens after peripheral nerve transection and subsequent regeneration.Journal of Neurocytology 18, 695–710.
Perez, V. J. &Moore, B. W. (1968) Wallerian degeneration in rabbit tibial nerve: changes in amounts of the S-100 protein.Journal of Neurochemistry 15, 971–7.
Pfenninger, K. H. &Johnson, M. P. (1983) Membrane biogenesis in the sprouting neuron. I. Selective transfer of newly synthesised phospholipid into the growing neurite.Journal of Cell Biology 87, 1038–42.
Ramon Y Cajal, S. (1928)Degeneration and Regeneration in the Nervous System. London: Oxford University Press.
Skene, J. H. P. (1989) Axonal growth-associated proteins.Annual Review of Neuroscience 12, 127–56.
Skene, J. H. P. &Virag, I. (1989) Posttranslational membrane attachment and dynamic fatty acylation of a neuronal growth cone protein, GAP-43.Journal of Cell Biology 108, 613–24.
Skene, J. H. P. &Willard, M. (1981a) Changes in axonally transported proteins during axon regeneration in toad retinal ganglion cells.Journal of Cell Biology 89, 86–95.
Skene, J. H. P. &Willard, M. (1981b) Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous system.Journal of Cell Biology 89, 96–103.
Skene, J. H. P., Jacobson, R. D., Snipes, G. J., McGuire, C. B., Norden, J. J. &Freeman, J. A. (1986) A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes.Science 233, 783–6.
Somogyi, P. &Takagi, H. (1982) A note on the use of picric acid-paraformaldehyde-glutaraldehyde fixative for correlated light and electron microscopic immunocytochemistry.Neuroscience 7, 1779–83.
Spreca, A., Rambotti, M. G., Rende, M., Saccardi, C., Aisa, M. C., Giambanco, I. &Donato, R. (1989) Immunocytochemical localization of S-100b protein in degenerating and regenerating rat sciatic nerves.Journal of Histochemistry and Cytochemistry 37, 441–6.
Stefansson, K., Wollmann, R. L. &Moore, B. W. (1982) Distribution of S-100 protein outside the central nervous system.Brain Research 234, 309–17.
Tetzlaff, W. &Bisby, M. A. (1989) Neurofilament elongation into regenerating facial nerve axons.Neuroscience 29, 659–66.
Tetzlaff, W., Zweirs, H., Lederis, K., Cassar, L. &Bisby, M. A. (1989) Axonal transport and localisation of B-50/GAP-43-like immunoreactivity in regenerating sciatic and facial nerves of the rat.Journal of Neuroscience 9, 1303–13.
Van Lookeren Campagne, M., Oestreicher, A. B., Van Bergen En Henegouwen, P. M. P. &Gispen, W. H. (1989) Ultrastructural immunocytochemical localization of B-50/GAP43, a protein kinase C substrate, in isolated presynaptic nerve terminals and neuronal growth cones.Journal of Neurocytology 18, 479–89.
Van Lookeren Campagne, M., Oestreicher, A. B., Van Der Krift, T. P., Gispen, W. H. &Verkleij, A. J. (1991) Freeze-substitution and Lowicryl HM20 embedding of fixed rat brain: suitability for immunogold ultrastructural localization of neural antigens.Journal of Histochemistry and Cytochemistry 39, 1267–79.
Verhaagen, J., Van Hooff, C. O. M., Edwards, P. M., De Graan, P. N. E., Oestreicher, A. B., Schotman, P., Jennekens, F. G. I. &Gispen, W. H. (1986) The kinase C substrate protein B-50 and axonal regeneration.Brain Research Bulletin 17, 737–41.
Vitković, L., Steisslinger, H. W., Aloyo, V. J. &Mersel, M. (1988) The 43-kDa neuronal growth-associated protein (GAP-43) is present in plasma membranes of rat astrocytes.Proceedings of the National Academy of Science (USA) 85, 8296–300.
Weinberg, H. J. &Spencer, P. S. (1978) The fate of Schwann cells isolated from axonal contact.Journal of Neurocytology 7, 555–69.
Woolf, C. J., Reynolds, M. L., Molander, C., O'Brien, C., Lindsay, R. M. &Benowitz, L. I. (1990) The growth-associated protein GAP-43 appears in dorsal root ganglion cells and in the dorsal horn of the rat spinal cord following peripheral nerve injury.Neuroscience 34, 465–78.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hall, S.M., Kent, A.P., Curtis, R. et al. Electron microscopic immunocytochemistry of GAP-43 within proximal and chronically denervated distal stumps of transected peripheral nerve. J Neurocytol 21, 820–831 (1992). https://doi.org/10.1007/BF01237907
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01237907