Abstract
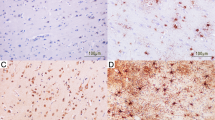
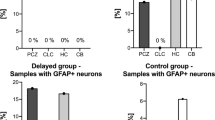
A neuropathological study of 41 forensic autopsy cases of hypoxic/ischemic brain damage has been undertaken, using immunohistochemical staining to detect the 70-kDa heat shock protein (hsp70) and the status of the glial cells. In cases surviving 2–5 h after hypoxic/ischemic injury, ischemic cell changes were seen whereas glial reactions were not apparent. In cases of longer survival, neuronal necrosis and a loss of neurons were seen, and these changes were accompanied by proliferation of glial fibrillary acidic protein (GFAP), vimentin-positive astrocytes and microglia which transformed into rod cells or lipid-laden macrophages. In cases with a history of hypoxic attacks, GFAP-positive and vimentin-negative astrocytes had proliferated in the CA3 and CA4 regions of hippocampus. The cases of severe hypoxic injury, such as an asthmatic attack and choking, showed no ischemic changes in the hippocampul neurons. On the other hand, the CA1 pyramidal cells showed neuronal necrosis in a patient suffering from tetralogy of Fallot (TOF), who survived for 2 h after a traffic accident. Therefore, it is suggested that even moderate hypoxic injury induces astrocytosis in the CA3 and CA4 regions and may affect the neuronal proteins and the metabolism, and that in cases with a history of hypoxic attacks neuronal damage may be severe even several hours after ischemic injury. The protein hsp70 expression was found in the CA2, CA3 and CA4 regions in cases of long-term survival after severe hypoxic/ischemic injury and in cases of alcoholic intake or toluene abuse just before acute death. Thus, it is suggested that the detection of hsp70 in the hippocampus indicates hypoxic/ischemic injury or other stress prior to death. In forensic practice, immunohistochemical investigation of the hsp70 and glial cell staining can be of great value for diagnosing not only hypoxic/ischemic brain damage during the process of death but also the victim's past history of hypoxic attacks.
Zusammenfassung
Eine neuropathologische Studie von 41 forensischen Autopsie-Fällen mit hypoxischen/ischämischen Hirnschäden wurde durchgeführt, um das 70-kDA Hitzeschock-Protein (hsp70) und den Zustand der Gliazellen zu untersuchen. In Fällen, in denen der hypoxisch-ischämische Schaden 2–5 Stunden überlebt wurde, waren ischämische Schäden erkennbar, während Glia-Reaktionen noch nicht vorhanden waren. In Fällen längerer Überlebenszeit war ein neuronale Nekrose und ein Verlust von Neuronen zu beobachten, und diese Veränderungen waren begleitet von einer Proliferation des glialen fibrillären sauren Proteins (GFAP), der Vimentin-positiven Astrozyten und der Mikro-Glia, welche in stabförmige Zellen oder lipidbeladene Makrophagen transformierte. In Fällen mit einer Anamnese von hypoxischen Attacken war eine Proliferation GFAP-positiver und Vimentin-negativer Astrozyten in der CA3- und CA4-Region des Hippocampus zu beobachten. Die Fälle mit schwerem hypoxschämischen Schaden, wie Asthma-Anfall und Strangulation, zeighten keine ischämischen Veränderungen in den Neuronen des Hippocampus. Andererseits zeigten die CA 1-Pyramiden-Zellen bei einem Patienten mit Fallot'scher Tetralogie (TOF), welcher zwei Stunden nach einem Verkehrsunfall starb, eine neuronale Nekrose. Daher wird vermutet, daß auch weniger schwere hypoxische Schäden eine Astrozytose in der CA3- und CA4-Region induzieren und einen Einfluß haben dürften auf die neuronalen Proteine und auf den Metabolismus und daß in Fällen mit einer Anamnese hypoxischer Attacken der neuronale Schaden schwer sein kann, sogar mehrere Stunden nach dem ischämischen Schaden. Das Protein hsp70 wurde in den CA2-, CA3- und CA4-Regionen in Fällen langzeitigen Überlebens nach schweren hypoxischen/ischämischen Schäden gefunden und in Fällen, in denen kurz vor dem Tode eine Alkoholaufnahme oder Toluol-Mißbrauch stattfand. Daher wird vermutet, daß ein Nachweis hsp70 im Hypocampus eine hypoxischen Schaden oder einen anderen Streß kurz vor dem Tode an zeigt. In der forensischen Praxis sind die immunhisto chemische Untersuchung von hsp70 und Gliazell-Fär bungen von großer Bedeutung für die Diagnostik nicht nur des hypoxisch-ischämischen Hirnschadens während des Sterbeprozesses, sondern auch für die Diagnostik der Anamnese des Opfers im Hinblick auf hypoxische At tacken.
Similar content being viewed by others
References
Brierley JB, Meldrum BS, Brown AW (1973) The threshold and neuropathology of cerebral “anoxic-ischemic” cell change. Arch Neurol 29: 367–374
Compton JL, MaCarthy BJ (1978) Induction of the Drosophila heat shock response in isolated polytene nuclei. Cell 14: 191–201
du Bois M, Bowman PD, Goldstein GW (1985) Cell proliferation after ischemic injury in gerbil brain. An immunocytochemical and autoradiographic study. Cell Tissue Res 242: 17–23
Gehrmann J, Bonnekoh P, Miyazawa T,Hossmann KA, Kreutzberg GW (1992) Immunocytochemical study of early microglial activation in ischemia. J Cereb Blood Flow Metab 12:257–269
Graham DI (1992) Hypoxia and vascular disorders. In: Adams JH, Duchen LW (eds) Greenfield's neuropathology. Edward Arnold, pp 153–268
Hirano A (1986) A guide to neuropathology. Igaku-shoin, Tokyo
Hom M, Schlote W (1992) Delayed neuronal death and delayed neuronal recovery in the brain following global ischemia. Acta Neuropathol 85:79–87
Kato H, Liu Y, Araki T, Kogure K (1991) Temporal profile of the effects of pretreatment with brief cerebral ischemia on the neuronal damage following secondary ischemic insult in the gerbil: cumulative damage and protective effects. Brain Res 553:238–242
Kirino T (1982) Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239:57–69
Kirino T, Sano K (1984 a) Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol 62:201–208
Kirino T, Sano K (1984b) Fine structural nature of delayed neuronal death following ischemia in the gerbil hippocampus. Acta Neuropathol 62:209–218
Kirino T, Tsujita Y, Tamura A (1991) Induced tolerance to ischemia in gerbil hippocampal neuron. J Cereb Blood Flow Metab 11:299–307
Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikosiba K, Kamada T (1990) ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res 528:21–24
Kwei S, Jiang C, Haddad GG (1993) Acute anoxia-induced alterations in MAP2 immunoreactivity and neuronal morphology in rat hippocampus. Brain Res 620:203–210
Li G (1983) Induction of thermotolerance and enhanced heat shock protein synthesis in Chinese hamster fibroblasts by sodium arsenite and by ethanol. J Cell Physiol 115:116–122
Lim R (1988) Glia maturation factor: an update. In: Norenberg M, Hertz L, Schousboc A(eds) The biochemical pathology of astrocytes (Neurology and neurobiology vol 39). Alan R. Liss, New York, pp 67–78
Mannoji H, Yeger H, Becker LE (1986) A specific histochemical marker (lectin Ricinus communis agglutinin-1) for normal human microglia, and application to routine histopathology. Acta Neuropathol 71: 341–343
Matsumoto M, Yamamoto K, Homburger HA, Yanagihara T (1987) Early detection of cerebral ischemic damage and repair process in the gerbil by use of an immunohistochemical technique. Mayo Clin Proc 62:460–472
Merril JE, Kutsunai S, Mohlstrom C, Hofman F, Groopman L, Golde DW (1984) Proliferation of astroglia and oligodendroglia in response to human T cell-derived factors. Science 224:1428–1430
Meyer FB (1989) Calcium, neuronal hyperexcitability and ischemic injury. Brain Res Brain Res Rev 14:227–243
Morioka T, Kalehua AN, Streit WJ (1991) The microglial reaction in the rat dorsal hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab 11: 966–973
Nagagomi T, Kirino T, Kanemitsu H, Tsujita Y, Tamura A (1993) Early recovery of protein synthesis following ischemia in hippocampal neurons with induced tolerance in the gerbil. Acta Neuropathol 86:10–15
Ng T, Graham DI, Adams JH, Ford I (1989) Changes in the hippocampus and the cerebellum resulting from hypoxic insults: frequency and distribution. Acta Neuropathol 78:438–443
O'Callaghan JP, Brinton RE, MacEwen BS (1989) Glucocorticoids regulate the concentration of glial fibrillary acidic protein throughout the brain. Brain Res 494: 159–161
Petito CK, Feldmann E, Pulsinelli WA, Plum F (1987) Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology 37:1281–1286
Petito CK, Morgello S, Felix JC, Holden LM (1988) Astrocytes in cerebral ischemia. In: Norenberg MD, Hertz L, Schousboe A (eds) The biochemical pathology of astrocytes (Neurology and neurobiology, vol 39). Alan R. Liss, New York, pp 341–349
Petito CK, Morgello S, Felix JC, Lesser ML (1990) The two patterns of reactive astrocytosis in postischemic rat brain. J Cereb Blood Flow Metab 10: 850–859
Sawada M, Suzunura A, Ohno K, Marunouchi T (1993) Regulation of astrocyte proliferation by prostaglandin E2 and the a subtype of protein kinase C. Brain Res 613:67–73
Schneider M (1961) Survival and revival of the brain in anoxia and ischemia. In: Meyer JS, Gastaut H (eds) Cerebral anoxia and the encephalogram. Charles C Thomas, Springfield, pp 134–143
Schlesinger M (1986) Heat shock protein: the search for functions. J Cell Biol 103: 321–325
Sciandra JJ, Subjeck JR, Hughes CS (1984) Induction of glucose-regulated proteins during anaerobic exposure and of heat shock proteins after reoxygenation. Proc Natl Acad Sci USA 81:4843–4847
Smith ML, Auer RN, Siesjö BK (1984) The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia. Acta Neuropathol 64:319–332
Tranque PA, Suarez I, Olmos G, Fernandez B, Garcia-Segura LM (1987) Estradiol — induced redistribution of glial fibrillary acidic protein immunoreactivity in the rat brain. Brain Res 406: 348–351
Takeda S, Ikuta F (1992) Neuronal vulnerability to hypoxia and hypoglycemia. Adv Neurol Sci 36:236–260 (in Japanese with English abstract)
Vass K, Welch WJ, Nowak TS Jr (1988) Localization of 70kDa stress protein induction in gerbil brain after ischemia. Acta Neuropathol 77: 128–135
Yamamoto K, Yoshimine T, Homburger HA, Yanagihara T (1986) Immunohistochemical investigation of regional cerebral ischemia in the gerbil: occlusion of the posterior communicating artery. Brain Res 371:244–252
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kitamura, O. Immunohistochemical investigation of hypoxiclischemic brain damage in forensic autopsy cases. Int J Leg Med 107, 69–76 (1994). https://doi.org/10.1007/BF01225492
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01225492