Abstract
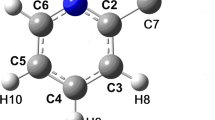
The crystal structure of 2-aminophenol has been redetermined from automatic diffractometer data in order to locate the hydroxyl and amino hydrogens and confirm the nature of the intermolecular hydrogen bonding. Crystals are orthorhombic, space groupPbca, witha =19.751(6),b = 7.250(3), andc = 7.852(5) Å, and eight molecules per cell. The structure was solved by Multan and refined to a conventionalR value of 0.030. The molecular geometry compares well with previously reported structures of related species. The molecules are hydrogen bonded from OH to N to form chains parallel to theb axis, with these chains linked together to form sheets by NH to O hydrogen bonds essentially parallel toc. The NH - O hydrogen bonds are 0.33 Å longer than the OH - N ones, and an explanation for this is provided which incorporates newly computed values for atomic electronegativities. Pairs of molecules are mutually hydrogen bonded across inversion centers. There are large cavities between the sheets which could conceivably result in easy intercalation of small foreign molecules, much as in the clathrates formed by urea and β-quinol.
Similar content being viewed by others
References
Ashfaquzzaman, S., and Pant, A. K. (1979)Acta Cryst. B 35, 1394–1399.
Bacon, G. E., and Jude, R. J. (1973)Z. Kristallogr. 138, 19–40.
Bois, C. (1972)Acta Cryst. B 28, 25–31.
Brown, C. J. (1951)Acta Cryst. 4, 100–103.
Brown, I. D. (1976)Acta Cryst. B 32, 24–31.
Cesur, A. F., and Richards, J. P. G. (1965)Z. Kristallogr. 122, 283–297.
Chao, M., and Schempp, E. (1977)Acta Cryst. B 33, 1557–1564.
Cox, E. G., Cruickshank, D. W. J., and Smith, J. A. S. (1958)Proc. R. Soc. London (A)247, 1–21.
Cromer, D. T., and Mann, J. B. (1968)Acta Cryst. A 24, 321–324.
DeRango, C., Brunie, S., Tsoucaris, G., Declercq, J. P., and Germain, G. (1974)Cryst, Struct. Commun. 3, 485–487.
Germain, G., Main, P., and Woolfson, M. M. (1971)Acta Cryst. A 27, 368–376.
Matcha, R. L. (1980) Private communication.
Palin, D. E., and Powell, H. M. (1948)J. Chem. Soc. 815–821.
Schlenk, W. (1949)Ann. Chem. 565, 204–240.
Sheldrick, G. M. (1976) Shelx 76. Program for Crystal Structure Determination, Univeristy of Cambridge, England.
Skapski, A. C., and Stevenson, J. L. (1973)J. Chem. Soc. Perkin Trans. 2 1197–1200.
Stalhandske, C. (1974)Acta Crysl. B 30, 1586–1589.
Stewart, R. F., Davidson, E. R., and Simpson, W. T. (1965)J. Chem. Phys. 42, 3175–3187.
Wallwork, S. C. (1962)Acta Cryst. 15, 758–759.
Whuler, A., Brouty, C., and Spinat, P. (1980)Acta Cryst. B 36, 1267–1269.
Wunderlich, H., and Mootz, D. (1971)Acta Cryst. B 27, 1684–1686.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Korp, J.D., Bernal, I., Aven, L. et al. A Redetermination of the crystal structure of 2-aminophenol. Journal of Crystal and Molecular Structure 11, 117–124 (1981). https://doi.org/10.1007/BF01210386
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01210386