Abstract
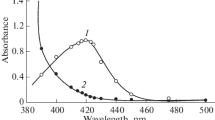
A sensitive spectrophotometric method is developed for the determination of small amounts of molybdenum based on the extraction of molybdenum-thiocyanate-4-acetyl-2-(acetylamino)-5-dimethyl-Δ 2-1,3,4-thiadiazole complex into chloroform from hydrochloric acid medium which is orange red in colour. The complex has an absorption maximum at 470 nm with a molar absorptivity of 2.01×104l·mole−1·cm−1. Beer's law is valid over the concentration range 0.06–2.5 ppm of molybdenum with an optimum concentration range of 0.15–2.2 ppm. The ternary complex is stable for over one week at room temperature. Equilibrium shift method indicates 1∶4∶2 composition for molybdenum-thio-cyanate-4-acetyl-2-(acetylamino)-5-dimethyl-Δ 2-1,3,4-thiadiazole complex. The effects of acidity, reagent concentrations, time, temperature and diverse ions upon the absorbance of the complex are critically assessed. This method has been used successfully for the determination of molybdenum in molybdenum steels.
Similar content being viewed by others
References
E. B. Sandell,Colorimetric Determination of Traces of Metals, Interscience, New York, 1959.
D. F. Boltz, M. G. Melon,Anal. Chem. 1976,48, 221R;1974,46, 234R;1972,44, 300R;1970,42, 152R;1968,40, 255R;1966,38, 317R.
J. A. Howie, L. G. Hargis,Anal. Chem. 1978,50, 243R.
M. M. L. Khosla, S. P. Rao,Anal. Chim. Acta 1971,57, 323.
C. P. Savariar, M. K. Arunachalam, T. R. Hariharan,Anal. Chim. Acta 1974,69, 305.
H. P. Tarasiewicz, A. Grudniewska, M. Tarasiewicz,Anal. Chim. Acta 1977,94, 435.
I. Adamiec,Chem. Anal. Warsaw 1966,1175, 1183.
A. I. Lazarev, V. I. Lazareva,Zavodsk. Lab. 1958,24, 798.
K. N. Thimmaiah, G. T. Chandrappa, W. D. Lloyd, C. Parkanyi,Inorg. Chim. Acta 1985,107, 1.
K. N. Thimmaiah, G. T. Chandrappa, W. D. Lloyd,Inorg. Chim. Acta 1985,107, 281.
S. Kubota, Y. Ueda, K. Fujikane, K. Toyooka, M. Shibuya,J. Org. Chem. 1980,45, 1473.
A. I. Vogel,Quantitative Inorganic Analysis, The Elbs Longmans, London, 1968, pp. 506.
J. Blazek, V. Mares,Chem. Abstr. 1967,66, 22266f.
Y. B. Kletinik, I. A. Bykhovskaya, L. V. Sekretox,Anal. Abstr. 1970,19, 3873.
V. T. Solomation, P. Y. Yokolev,Chem. Abstr. 1975,83, 21546m.
V. P. R. Rao, Y. Anjaeyulu, A. S. R. Murthy,Mikrochim. Acta [Wien] 1975, 265.
A. T. Pilipenko, Z. G. Solomenia,Chem. Abstr. 1974,80, 66382j.
B. Tamhina, M. J. Herak,Mikrochim. Acta [Wien] 1976,553.
H. Puzanowska, A. Tarasiewicz, A. Grudniewska, M. Tarasiewicz,Anal. Chim. Acta 1977,94, 440.
J. Bjerrum,Metal Amine Formation in Aqueous Solution, Hasse, Copenhagen, 1941, p. 298.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thimmaiah, K.N., Chandrappa, G.T. & Sekhar, V.C. Extraction spectrophotometric investigation of mixed ligand complex of molybdenum(V) with thiocyanate and 4-acetyl-2-(acetylamino)-5-dimethyl-Δ2-1,3,4-thiadiazole. Mikrochim Acta 90, 277–285 (1986). https://doi.org/10.1007/BF01199270
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01199270