Summary
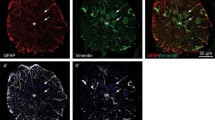
Lesion-induced regenerative sprouting of CNS axons is accompanied by structural and metabolic changes of astrocytes. In order to evaluate the effects of these astrocytic changes on axonal regeneration, we investigated the spatio-temporal relationship of gliosis, laminin expression and axonal sprouting in the postcommissural fornix of the adult rat. Using immunocytochemical methods we observed (1) a perilesional area with a transient lack of astrocytes and axons, (2) the reappearance of reactive astrocytes followed by the ingrowth of sprouting fibres and finally an increase in laminin-immunoreactivity, (3) the absence of lesion-induced laminin-expression in reactive astrocytes and (4) the formation and long-lasting (at least 28 months) persistence of a dense plexus of laminin-immunopositive blood vessels at the site of transection and in the proximal and distal stumps. These data indicate that astrogliosis is permeable for regrowing axons and that injury-induced axonal sprouting in the transected postcommissural fornix may be mediated by laminin-independent mechanisms.
Similar content being viewed by others
References
Alonso, G. &Privat, A. (1993a) Neuropeptide Y-producing neurons of the arcuate nucleus regenerate axons after surgical deafferentiation of the mediobasal hypothalamus.Journal of Neuroscience Research 34, 510–22.
Alonso, G. &Privat, A. (1993b) Reactive astrocytes involved in the formation of lesional scars differ in the mediobasal hypothalamus and in other forebrain regions.Journal of Neuroscience Research 34, 523–38.
Ard, M. D. &Bunge, R. P. (1986) Tissue culture observations on the interactions of astrocytes, extracellular matrix and neurites.Society for Neuroscience Abstracts 12, 395.
Ard, M. D. &Faissner, A. (1991) Components of astrocytic extracellular matrix are regulated by contact with axons.Annals of the New York Academy of Sciences 633, 566–9.
Bähr, M. (1991) Adult rat retinal gliain vitro: effects ofin vivo crush-activation on glia proliferation and permissiveness for regenerating retinal ganglion cell axons.Experimental Neurology 111, 65–73.
Bernstein, J. J. &Bernstein, M. E. (1971) Axonal regeneration and formation of synapses proximal to the site of lesion following hemisection of the rat spinal cord.Experimental Neurology 30, 336–51.
Bernstein, J. J., Getz, R., Jefferson, M. &Kelemen, M. (1985) Astrocytes secrete basal lamina after hemisection of rat spinal cord.Brain Research 327, 135–41.
Berry, M., Hall, S., Follows, R., Rees, L., Gregson, N. &Sievers, J. (1988) Response of axons and glia at the site of anastomosis between the optic nerve and cellular and acellular sciatic nerve grafts.Journal of Neurocytology 17, 727–44.
Berry, M., Hall, S., Rees, L., Carlile, J. &Wyse, J. P. H. (1992) Regeneration of axons in the optic nerve of the adult Browman-Wyse (BW) mutant rat.Journal of Neurocytology 21, 426–48.
Björklund, A., Katzman, R., Stenevi, U. &West, K. A. (1971) Development and growth of axonal sprouts from noradrenalin and 5-hydroxytryptamine neurones in the rat spinal cord.Brain Research 31, 21–33.
Björklund, A. &Stenevi, U. (1979) Regeneration of monoaminergic and cholinergic neurons in the central nervous system.Physiological Reviews 59, 62–100.
Blaugrund, E., Duvdevani, R., Lavie, V., Solomon, A. &Schwartz, M. (1992) Disappearance of astrocytes and invasion of macrophages following crush injury of adult rodent optic nerves: implications for regeneration.Experimental Neurology 118, 105–15.
Blaugrund, E., Lavie, V., Cohen, I., Solomon, A., Schreyer, D. J. &Schwartz, M. (1993) Axonal regeneration is associated with glial migration: comparison between the injured optic nerves of fish and rats.Journal of Comparative Neurology 330, 105–12.
Bovolenta, P., Wandosell, F. &Nieto-Sampedro, M. (1993) Characterization of neurite outgrowth inhibitor expressed after CNS injury.European Journal of Neuroscience 5, 454–65.
Carlstedt, T. (1985) Regenerating axons form nerve terminals at astrocytes.Brain Research 347, 188–91.
Cheng, C. T., Zhou, F. C. &Lee, C. H. (1989) Laminin expression in developmental rat brain: a quantitativein situ hybridization study using image analyzer.Society for Neuroscience Abstracts 15, 278.
Clemente, C. D. (1955) Structural regeneration in the mammalian central nervous system and the role of neuroglia and connective tissue. In:Regeneration in the Central Nervous System (edited byWindle, W. F.) pp. 147–61. Springfield, IL: Charles C. Thomas.
Cohen, J., Burne, J. F., Winter, J. &Bartlett, P. (1986) Retinal ganglion cells lose response to laminin with maturation.Nature 322, 465–7.
David, S. (1988) Neurite outgrowth from mammalian CNS neurons on astrocytes in vitro may not be mediated primarily by laminin.Journal of Neurocytology 17, 131–44.
David, S., Bouchard, C., Tsatas, O. &Giftochristos, N. (1990) Macrophages can modify the nonpermissive nature of the adult mammalian central nervous system.Neuron 5, 463–9.
Dellmann, H. D. (1973) Degeneration and regeneration of neurosecretory systems.International Reviews of Cytology 36, 215–315.
Eddleston, M. &Mucke, L. (1993) Molecular profile of reactive astrocytes — implications for their role in neurological disease.Neuroscience 54, 15–36.
Edgar, D., Timpl, R. &Thoenen, H. (1984) The heparinbinding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival.EMBO Journal 3, 1463–8.
Engvall, E., Davis, G. E., Dickerson, K., Ruoslahti, E., Varon, S. &Manthorpe, M. (1986) Mapping of domains in human laminin using monoclonal antibodies: localization of neurite-promoting site.Journal of Cell Biology 103, 2457–65.
Eriksdotter-Nilsson, M., Björklund, H. &Olson, L. (1986) Laminin immunohistochemistry: a simple method to visualize and quantitate vascular structures in the mammalian brain.Journal of Neuroscience Methods 17, 275–86.
Eriksdotter-Nilsson, M., Björklund, H., Dahl, D., Olson, L. &Ingvar, M. (1987) Sustained seizures cause circumscribed cerebral changes in glial fibrillary acidic protein, neurofilament and laminin immunofluorescence.Experimental Brain Research 69, 155–66.
Fawcett, J. W. (1989) Oligodendrocytes repel axons and cause axonal growth cone collapse.Journal of Cell Science 92, 93–100.
Feringa, E. R., Kowalski, T. F. &Vahlsing, H. L. (1980) Basal lamina formation at the site of spinal cord transection.Annals of Neurology 8, 148–54.
Fernaud-Espinosa, I., Nieto-Sampedro, M. &Bovolenta, P. (1993) Differential activation of microglia and astocytes in aniso- and isomorphic gliotic tissue.Glia 8, 277–91.
Frisen, J., Fried, K., Sjögren, M. &Risling, M. (1993) Growth of ascending spinal axons in CNS scar tissue.International Journal of Developmental Neuroscience 11, 461–75.
Gasser, U. E. &Hatten, M. E. (1990) Neuron-glia interactions of developing rat hippocampal cellsin vitro: glial-guided neuronal migration and neuronal regulation of glial differentiation.Journal of Neuroscience 10, 1276–85.
Giftochristos, N. &David, S. (1988) Laminin and heparan sulphate proteoglycan in the lesioned adult mammalian central nervous system and their possible relationship to axonal sprouting.Journal of Neurocytology 17, 385–97.
Gocht, A. &Löhler, J. (1993) Microenvironmental changes during axonal regrowth in the optic nerve of the myelin deficient rat. Immunocytochemical and ultrastructural observations.Journal of Neurocytology 22, 461–79.
Grabber, M. B., Streit, W. J. &Kreutzberg, G. W. (1988) The microglial cytoskeleton: vimentin is localized within activated cellsin situ.Journal of Neurocytology 17, 573–80.
Graziadei, P. P. D. &Monti Graziadei, G. A. (1978) The olfactory system: a model for the study of neurogenesis and axon regeneration in mammals. In:Neuronal Plasticity (edited byCotman, C. W.) pp. 113–53. New York: Raven Press.
Hagg, T., Muir, D., Engvall, E., Varon, S. &Manthorpe, M. (1989) Laminin-like antigen in rat CNS neurons: distribution and changes upon brain injury and nerve growth factor treatment.Neuron 3, 721–32.
Hall, S., Gregson, N. &Rickard, S. (1991) Interaction of regrowing PNS axons with transplanted aggregates of cultured CNS gliain vivo.Journal of Neurocytology 20, 299–309.
Hatten, M. E. (1985) Neuronal regulation of astroglial morphology and proliferation in vitro.Journal of Cell Biology 100, 384–96.
Hatten, M. E., Liem, R. K. H., Shelanski, M. L. &Mason, C. A. (1991) Astroglia in CNS injury.Glia 4, 233–43.
Hausmann, B., Sievers, J., Hermanns, J. &Berry, M. (1989) Regeneration of axons from the adult rat optic nerve: influence of fetal brain grafts, laminin and artificial basement membrane.Journal of Comparative Neurology 281, 447–66.
Hu, C. T., Zhou, F. C., Hwang, Y. W. &Lee, C. H. (1988) Laminin expression in the developmental rat brain: an immunocytochemical andin situ-hybridization study.Society for Neuroscience Abstracts 14, 364.
Hunter, D. D., Llinas, R., Ard, M., Merlie, J. P. &Sanes, J. R. (1992) Expression of S-laminin and laminin in the developing rat central nervous system.Journal of Comparative Neurology 323, 238–51.
Jucker, M., Kleinman, H. K., Höhmann, C. F., Ordy, J. M. &Ingram, D. K. (1991) Distinct immunoreactivity to 110 kDa laminin-binding protein in adult and lesioned rat forebrain.Brain Research 555, 305–12.
Jucker, M., Bialobok, P., Hagg, T. &Ingram, D. K. (1992) Laminin immunohistochemistry in brain is dependent on method of tissue fixation.Brain Research 586, 166–70.
Kao, C. C., Chang, L. W. &Bloodworth, J. M. B.Jr. (1977) Axonal regeneration across transected mammalian spinal cords: an electron microscopic study of delayed microsurgical nerve grafting.Experimental Neurology 54, 591–615.
Kawaja, M. D., Rosenberg, M. B., Yoshida, K., Friedman, T. &Gage, F. H. (1990) Axonal sprouting induced by striatal implants of genetically modified fibroblasts that produce nerve growth factor.Society for Neuroscience Abstracts 16, 814.340.4
Kiernan, J. A. (1979) Hypotheses concerned with axonal regeneration in the mammalian nervous system.Biological Reviews 54, 155–97.
Kleinman, H. K., McGarvey, M. L., Liotta, L. A., Robey, P. G., Tryaason, K. &Martin, G. R. (1985) Isolation and characterization of type IV procollagen, laminin and heparan sulfate proteoglycan from the EHS sarcoma.Biochemistry 21, 6188–93.
Lawrence, J. M., Huang, S. K. &Raisman, G. (1984) Vascular and astrocytic reactions during establishment of hippocampal transplants in adult host brain.Neuroscience 12, 745–60.
Laywell, E. D., Dörries, U., Bartsch, U., Faissner, A. &Schachner, M. (1992) Enhanced expression of the developmentally regulated extracellular matrix molecule tenascin following adult brain injury.Proceedings of the National Academy of Sciences (USA) 89, 2634–8.
Liesi, P. (1985) Laminin-immunoreactive glia distinguish regenerative adult CNS systems from non-regenerating ones.EMBO Journal 4, 2505–11.
Liesi, P., Kaakkola, S., Dahl, D. &Vaheri, A. (1984) Laminin is produced in astrocytes of adult brain by injury.EMBO Journal 3, 683–6.
Liuzzi, F. J. &Lasek, R. J. (1987) Astrocytes block axonal regeneration by activating the physiological stop pathway.Science 237, 642–64.
Mansour, H., Asher, R., Dahl, D., Labkovsky, B., Perides, G. &Bignami, A. (1990) Permissive and nonpermissive reactive astrocytes: immunofluorescence study with antibodies to the glial hyaluronate-binding protein.Journal of Neuroscience Research 25, 300–11.
Manthorpe, M., Engvall, E., Ruoslahti, E., Longo, F. M., Dans, G. E. &Varon, S. (1983) Laminin promotes neuritic regeneration from cultured peripheral and central neurons.Journal of Cell Biology 97, 1882–90.
Matthiessen, H. P., Schmalenbach, C. &Müller, H. W. (1989) Astroglia-released neurite growth inducing activity for embryonic hippocampal neurons is associated with laminin bound in a sulfated complex and free fibronectin.Glia 2, 177–88.
McKeon, R. J., Schreiber, R. C., Rudge, J. S. &Silver, J. (1991) Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes.Journal of Neuroscience 11, 3398–411.
McLean, I. W. &Nakane, P. K. (1974) Periodate-lysineparaformaldehyde fixative. A new fixative for immunelectron microscopy.Journal of Histochemistry and Cytochemistry 22, 1077–83.
McLoon, S. C. (1986) Response of astrocytes in the visual system to Wallerian degeneration: an immunohistochemical analysis of laminin and glial fibrillary acidic protein (GFAP).Experimental Neurology 91, 613–21.
Neuberger, T. J., Cornbrooks, C. J. &Kromer, L. F. (1992) Effects of delayed transplantation of cultured Schwann cells on axonal regeneration of central nervous system cholinergic neurons.Journal of Comparative Neurology 315, 16–33.
Ogawa, M., Araki, M., Nagatsu, I. &Yoshida, M. (1989) Astroglial cell alteration caused by neurotoxins: immunohistochemical observations with antibodies to glial fibrillary acidic protein, laminin and tyrosine hydroxylase.Experimental Neurology 106, 187–96.
Pindzola, R. R., Doller, C. &Silver, J. (1993) Putative inhibitory extracellular matrix molecules at the dorsal root entry zone of the spinal cord during development and after root and sciatic nerve lesions.Developmental Biology 156, 34–48.
Rakic, P. (1981) Neuronal-glial interaction during brain development.Trends in Neurosciences 4, 184–7.
Ramon Ycajal, S. (1928)Degeneration and Regeneration of the Nervous System. New York: Hafner.
Reier, P. J. &Houle, J. D. (1988) The glial scar: its bearing on axonal elongation and transplantation approaches to CNS repair. In:Advances in Neurology: Functional Recovery of Neurological Disease, (edited byWaxman, S. G.) pp. 87–138. New York: Raven Press.
Reier, P. J., Stensaas, L. J. &Guth, L. (1983) The astrocytic scar as an impediment to regeneration in the central nervous system. In:Spinal Cord Reconstruction (edited byKao, C. C., Bunge, R. P. &Reier, P. J.) Vol. 47. pp. 163–96. New York: Raven Press.
Rogers, S. L., Letourneau, P. C., Palm, S. L., McCarthy, J. &Furcht, L. T. (1983) Neurite extension by peripheral and central nervous system neurons in response to substratum-bound fibronectin and laminin.Developmental Biology 98, 212–20.
Rudge, J. S. &Silver, J. (1990) Inhibition of neurite outgrowth on astroglial scarsin vitro.Journal of Neuroscience 10, 3594–603.
Schiffer, D. E., Giordana, M. T., Giaccone, G., Pezzotta, S. &Mauro, A. (1986) Glial fibrillary acidic protein and vimentin in the experimental glial reaction of the rat brain.Brain Research 374, 110–18.
Schmidt-Kastner, R., Wietasch, K., Weigel, H. &Eysel, U. T. (1993) Immunohistochemical staining for glial fibrillary acidic protein (GFAP) after deafferentiation or ischemic infarction in rat visual system: features of reactive and damaged astrocytes.International Journal of Developmental Neuroscience 11, 157–74.
Schwab, M. E. &Caroni, P. (1988) Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreadingin vitro.Journal of Neuroscience 8, 2381–93.
Schwab, M. E., Kapfhammer, J. P. &Bandtlow, C. E. (1993) Inhibitors of neurite growth.Annual Reviews of Neuroscience 16, 565–95.
Shigematsu, K., Kamo, H., Akiguchi, I., Kimura, J., Kameyama, M. &Kimura, H. (1989) Neovascularization in kainic acid-induced lesions of rat striatum: an immunohistochemical study with laminin.Brain Research 501, 215–22.
Smith, G. M., Rutishauser, U., Silver, J. &Miller, R. H. (1986) Changing role of forebrain astrocytes during development, regenerative failure, and induced regeneration upon transplantation.Journal of Comparative Neurology 251, 23–43.
Sosale, A., Robson, J. A. &Stelzner, D. J. (1988) Laminin distribution during corticospinal tract development and after spinal cord injury.Experimental Neurology 102, 14–22.
Stichel, C. C., Singer, W. &Zilles, K. (1990) Ultrastructure of PkC (II/III)-immunopositive structures in rat primary visual cortex.Experimental Brain Research 82, 575–84.
Stichel, C. C. &Müller, H. W. (1994) Extensive and long-lasting changes of glial cells following transection of the postcommissural fornix in the adult rat.Glia 10, 89–100.
Suzuki, H., Yamamoto, T., Yamamoto, H., Konno, H., Iwasaki, Y., Ohara, Y. &Terunuma, H. (1990) Intraneuronal laminin-like immunoreactivity in the human central nervous system.Brain Research 520, 324–9.
Takamiya, Y., Kohsaka, S., Toya, S., Otani, M. &Tsukada, Y. (1988) Immunohistochemical studies on the proliferation of reactive astrocytes and the expression of cytoskeletal proteins following brain injury in rats.Developmental Brain Research 38, 201–10.
Timple, R., Rohde, H., Robey, P. G., Rennard, S. I., Foidart, J.-M. &Martin, G. R. (1979) Laminin — a glycoprotein from basement membranes.Journal of Biological Chemistry 254, 9933–7.
Tomaselli, K. J., Reichardt, L. F. &Bixby, J. L. (1986) Distinct molecular interactions mediate neuronal process outgrowth on non-neuronal cell surfaces and extracellular matrices.Journal of Cell Biology 103, 2659–72.
Wilkin, G. P., Marriott, D. R. &Cholewinski, A. J. (1990) Astrocyte heterogeneity.Trends in Neuroscience 13, 43–6.
Wolburg, H. &Kästner, R. (1984) Is the architecture of astrocytic membrane crucial for axonal regeneration in the central nervous system?Naturwissenschaften 71, 484–5.
Wujek, J. R. &Reier, P. J. (1984) Astrocytic membrane morphology: differences between mammalian and amphibian astrocytes after axotomy.Journal of Comparative Neurology 222, 607–19.
Wunderlich, G., Stichel, C. C., Schroeder, W. &Müller, H. W. (1994) Transplants of immature astrocytes promote axonal regeneration in the adult rat brain.Glia 10, 49–58.
Yamamoto, T., Iwasaki, Y., Yamamoto, H., Konno, H. &Isemura, M. (1988) Intraneuronal laminin-like molecule in the central nervous system: demonstration of its unique differential distribution.Journal of the Neurological Sciences 84, 1–13.
Zhou, F. C. (1990) Four patterns of laminin-immunoreactive structure in developing rat brain.Developmental Brain Research 55, 191–201.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stichel, C.C., Müller, H.W. Relationship between injury-induced astrogliosis, laminin expression and axonal sprouting in the adult rat brain. J Neurocytol 23, 615–630 (1994). https://doi.org/10.1007/BF01191556
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01191556