Summary
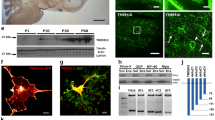
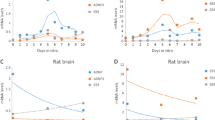
We have determined the cellular localization of the neurite outgrowth-promoting protein, amphoterin (p30), within the developing rat spinal cord in order to gain insight into possible function(s). Both neurons and oligodendrocytes are labelled by anti-amphoterin antibodies in tissue sections. Double labelling confirmed that labelled non-neuronal cells were oligodendrocytes and not astrocytes. White matter cells labelled by anti-amphoterin were first apparent in the spinal cord at embryonic day 18 (E18). The spinal cord white matter was most densely populated by amphoterin immunoreactive oligodendrocytes during the first postnatal week. In the adult spinal cord anti-amphoterin-labelled oligodendrocytes were infrequent. Neuronal staining was not diminished in adult animals. We have identified multiple populations of developing oligodendrocytes in the rat spinal cord. Oligodendrocytes (or precursors) in the presumptive white matter at E20 are elongated, radially oriented cells. Some are amphoterin+ and Rip−, while others are positive for both antigens. At the same age, a small number of process-bearing Rip+ cells are present in the grey matter. These morphologically and antigenically defined populations of cells could reflect different oligodendrocyte lineages, developmental stages, or different responses to environmental cues. While the function of amphoterin is unknown, the finding that amphoterin is expressed in numerous oligodendrocytes from late embryonic development suggests that amphoterin expression may be important in the early stages of oligodendrocyte maturation. The onset of amphoterin expression, prior to the expression of myelin-specific antigens, makes amphoterin a useful marker for studying oligodendrocyte development.
Similar content being viewed by others
References
Asbury, A. K. (1967) Schwann cell proliferation in developing mouse sciatic nerve.Journal of Cell Biology 34, 735–43.
Bignami, A., Eng, L. F., Dahl, D. &Uyeda, C. T. (1972) Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence.Brain Research 43, 429–35.
Bustin, M. &Neihart, N. K. (1979) Antibodies against chromosomal HMG proteins stain the cytoplasm of mammalian cells.Cell 16, 181–9.
Choi, B. H., Kim, R. C. &Lapham, L. W. (1983) Do radial glia give rise to both astroglial and oligodendroglial cells?Developmental Brain Research 8, 119–30.
Daston, M. M. &Ratner, N. (1991) Expression of P30, a protein with adhesive properties, in Schwann cells and neurons of the developing and regenerating peripheral nerve.Journal of Cell Biology 112, 1229–39.
Del Rio Hortega, P. (1921) La glia de escasas radiaciones (oligodendroglia).Boletin De La Real Sociedad Espanola De Historia Natural 21, 63–92.
Einck, L. &Bustin, M. (1983) Inhibition of transcription in somatic cells by microinjection of antibodies to chromosomal proteins.Proceedings of the National Academy of Sciences (USA) 80, 6735–9.
Friedman, B., Hockfield, S., Black, J. A., Woodruff, K. A. &Waxman, S. G. (1989)In situ demonstration of mature oligodendrocytes and their processes: an immunocytochemical study with a new monoclonal antibody, Rip.Glia 2, 380–90.
Goldman, J. E. &Vayasse, P. J.-J. (1991) Tracing glial lineages in the mammalian forebrain.Glia 4, 149–56.
Goodwin, G. H., Sanders, C. &Johns, E. W. (1973) Anew group of chromatin-associated proteins with a high content of acidic and basic amino acids.European Journal of Biochemistry 28, 14–19.
Hartman, B. K., Agrawal, H. C., Agrawal, D. &Kalmbach, S. (1982) Development and maturation of central nervous system myelin: comparison of immuno- histochemical localization of proteolipid protein and basic protein in myelin and oligodendrocytes.Proceedings of the National Academy of Sciences (USA) 79, 4217–20.
Hirano, M. &Goldman, J. E. (1988) Gliogenesis in rat spinal cord: evidence for origin of astrocytes and oligodendrocytes from radial precursors.Journal of Neuroscience Research 21, 155–67.
Jessen, K. R. &Mirsky, R. (1991) Schwann cell precursors and their development.Glia 4, 185–94.
Merenmies, J., Pihlaskari, R., Laitinen, J., Wartiovaara, J. &Rauvala, H. (1991) 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth.Journal of Biological Chemistry 266, 16722–9.
Mohan, P., Laitinen, J., Merenmies, J., Rauvala, H. &Jungalwala, F. B. (1992) Sulfoglycolipids bind to adhesive protein amphoterin (p30) in the nervous system.Biochemical and Biophysical Research Communications 182, 689–96.
Mosevitsky, M. I., Novitskaya, V. A., Iogannsen, M. G. &Zabezhinsky, M. A. (1989) Tissue specificity of nucleo-cytoplasmic distribution of HMG1 and HMG2 proteins and their probable functions.European Journal of Biochemistry 185, 303–10.
Noll, E. &Miller, R. H. (1993) Oligodendrocyte precursors originate at the ventral ventricular zone dorsal to the ventral midline region in the embryonic rat spinal cord.Development 118, 563–73.
Parkkinen, J. &Rauvala, H. (1991) Interactions of plasminogen and tissue plasminogen activator (t-PA) with amphoterin.Journal of Biological Chemistry 266, 16730–5.
Penfield, W. (1924) Oligodendroglia and its relation to classical neuroglia.Brain 47, 430–52.
Pringle, N. P. &Richardson, W. (1993) A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligo-dendrocyte lineage.Development 117, 525–33.
Rauvala, H. &Pihlaskari, R. (1987) Isolation and some characteristics of an adhesive factor of brain that enhances neurite outgrowth in central neurons.Journal of Biological Chemistry 262, 16625–35.
Rauvala, H., Merenmies, J., Pihlaskari, R., Korkolainen, M., Huhtala, M.-L. &Panula, P. (1988) The adhesive and neurite-promoting molecule p30: analysis of the amino-terminal sequence and production of antipeptide antibodies that detect p30 at the surface of neuroblastoma cells and of brain neurons.Journal of Cell Biology 107, 2292–305.
Remahl, S. &Hildebrand, C. (1990) Relations between axons and oligodendroglial cells during initial myelination. I. The glial unit.Journal of Neurocytology 19, 313–28.
Salmivirta, M., Rauvala, H., Elenius, K. &Jalkanen, M. (1992) Neurite growth-promoting protein (amphoterin, p30) binds syndecan.Experimental Cell Research 200, 444–51.
Schwab, M. E. &Schnell, L. (1989) Region-specific appearance of myelin constituents in the developing rat spinal cord.Journal of Neurocytology 18, 161–9.
Sternberger, N. H., Itoyama, Y., Kies, M. W. &Webster, H. Def. (1978) Myelin basic protein demonstrated immunocytochemically in oligodendroglia prior to myelin sheath formation.Proceedings of the National Academy of Sciences (USA) 75, 2521–4.
Sternberger, N. H., Quarles, R. H., Itoyama, Y. &Webster, H. Def. (1979) Myelin-associated glycoprotein demonstrated immunocytochemically in myelin and myelin-forming cells of developing rat.Proceedings of the National Academy of Sciences (USA) 76, 1510–14.
Teng, C. T. &Teng, C. S. (1981) Changes in quantities of high-mobility-group protein 1 in oviduct cellular fractions after oestrogen stimulation.Biochemical Journal 198, 85–90.
Tsuda, K., Kikuchi, M., Mori, K., Waga, S. &Yoshida, M. (1988) Primary structure of non-histone protein HMG1 revealed by the nucleotide sequence.Biochemistry 27, 6159–63.
Voigt, T. (1989) Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into ostrocytes.Journal of Comparative Neurology 289, 74–88.
Warf, B. C., Fok-Seang, J. &Miller, R. H. (1991) Evidence for the ventral origin of oligodendrocyte precursors in the rat spinal cord.Journal of Neuroscience 11, 2477–88.
Webster, H. Def, Martin, J. R. &O'connell, M. F. (1973) The relationships between interphase Schwann cells and axons before myelination: a quantitative electron microscopic study.Developmental Biology 32, 401–16.
Wen, L., Huang, J.-K., Johnson, B. H. &Reeck, G. R. (1989) A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1.Nucleic Acids Research 17, 1197–214.
Wood, P. &Bunge, R. P. (1984) The biology of the oligodendrocyte. In:Oligodendroglia (edited byNorton, W. T.), pp. 1–46. Plenum Press, New York, NY.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Daston, M.M., Ratner, N. Amphoterin (P30, HMG-1) and RIP are early markers of oligodendrocytes in the developing rat spinal cord. J Neurocytol 23, 323–332 (1994). https://doi.org/10.1007/BF01188500
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01188500