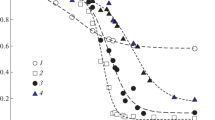
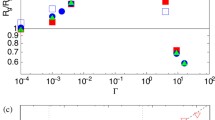
Abstract
The stability of a polyelectrolyte gel in solution results from a delicate balance between several competing thermodynamic forces, viz.
-
(i)
osmotic pressure of free ions in the gel,
-
(ii)
molecular interaction of solvent and polymer molecules,
-
(iii)
network elasticity,
-
(iv)
Debye-Htickel interaction of ions.
That balance may be upset by a decrease of temperature and by the addition of salt to the solvent. This results in a decrease of osmotic pressure and collapse of the gel to a small fraction of the initial volume. The effect can be reversed by increasing temperature and by removing salt from the solution. This paper presents an attempt to describe swelling and shrinking quantitatively and to understand the nature of the opposing forces. The volume of a particular polyacrylamide gel in a water acetone solution is represented as a function of the salt content and of temperature.
Similar content being viewed by others
References
Flory, J. P.: Principles of polymer chemistry. Ithaca, London: Cornell University Press 1953
Gardon, J. L.: Cohesve-energy density. In: Encyclopedia of Polymer Science and Technology. Vol. 3. New York, London, Sydney: Interscience Publishers 1965
Janas, V. F..; Rodriguez, F.; Cohen, C. Ageing and thermodynamics of polyacrylamide gels. Macromolecules. 13 (1980) 977–983
Münster, A.: Statistische Thermodynamik, pp. 767–773. Berlin, Göttingen, Heidelberg: Springer 1956
Overbeek, J. Th. G.: The Donnan equilibrium. Progress in Biophys. and Biophys. Chem. 6 (1956) 57–84
Katchalsky, A.: Polyelectrolyte gels. Progress in Biophys. and Biophys. Chem. 4 (1954) 1–59
Buchanan, K. J.; Hird, B.; Letcher, T. M.: Hydrogels. Polymer Bulletin 15 (1986) 325–332
Donnan, F. G.: Theorie der Membrangleichgewichte und Membranpotentiale bei Vorhandensein von nicht dialysierenden Elektrolyten. Z. Elektrochemie 17 (1911) 572–581
Rička, J.; Tanaka, T.: Swelling of ionic gels: Quantitative performance of the Donnan theory. Macromolecules 17 (1984) 2916–2921
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rydzewski, R. Swelling and shrinking of a polyelectrolyte gel induced by a salt solution. Continuum Mech. Thermodyn 2, 77–97 (1990). https://doi.org/10.1007/BF01126716
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01126716